PharmaTher (PHRM.C) announced today that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation (ODD) for ketamine in the treatment of Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s disease.
“Receiving FDA orphan drug designation is a massive validation for ketamine as a potential treatment for ALS, and it allows us to confidently proceed in evaluating ketamine in a phase 2 clinical study in patients suffering from this life-threatening disease,” said Fabio Chianelli, Chief Executive Officer of PharmaTher.
ALS is a neurological disease that primary affects the nerve cells associated with controlling voluntary muscle movements like walking, chewing, talking and, eventually, breathing. The disease itself is progressive, meaning the symptoms get worse over time as the upper and lower motor neurons die, causing the brain to lose its ability to control muscle movement.
It wasn’t until the ALS Ice Bucket Challenge began circulating online in 2014 that I started to understand the severity of ALS. Considering donations from the challenge enabled the ALS Associated to increase annual funding for research globally by 187%, I think its safe to say I was not the only one who came to this realization. From diagnosis, those with the neuromuscular disease have a life expectancy of only two to six years, Furthermore, there is no known cure for ALS, which affects approximately 50,000 people in the US and Europe. To make matters worse, more than 5,000 new cases are diagnosed annually and the only FDA approved pharmaceuticals available for the treatment of ALS include riluzole, edaravone, and Neudexta, none of which have a measurable effect on slowing disease progression or improving survival.
PharmaTher entered into an exclusive license agreement with The University of Kansas (KU) on March 2, 2021, for the development and commercialization for the intellectual property of ketamine in the treatment of ALS. Since then, the University of Kansas Medical Center’s researchers and inventors, namely Dr. Richard J. Barohn, M.D., John A. Stanford, Ph.D., and Dr. Matthew Macaluso, D.O., have made the discovery that ketamine can be https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered as an effective treatment for ALS. In particular, it was discovered that ketamine has the potential to effectively increase the life expectancy of those with ALS at any stage and slow progressive loss of muscle associated with poor outcomes of the disease.
“This designation not only expedites our regulatory, clinical and product development plans, but also validates our belief in the potential of ketamine as a therapeutic solution for neurological disorders,” continued Fabio Chianelli.
The FDA grants ODD to products that treat rare diseases, which are defined as those which affect fewer than 200,000 people in the US at any given time. With this in mind, an ODD would qualify a drug for certain benefits and incentives, including seven years of marketing exclusivity, potential tax credits for certain clinical drug testing costs, eligibility for grants, and the waiver of the FDA New Drug Application filing fee of approximately $2.4 million. Well, I am starting to see why some companies get pissed off when their NDAs get rejected…I mean, $2.4 million isn’t exactly chump change. Regardless, having now received an ODD, PharmaTher is well positioned to pursue ketamine as a viable treatment option for ALS.
As of February 28, 2021, PharmaTher’s total assets and total liabilities were CAD$7,106,120 and CAD$1,459,379, respectively. Furthermore, the Company’s cash increased significantly from CAD$388,382 on May 31, 2020 to CAD$3,639,367 as of February 28, 2021. In addition to the Company’s upcoming evaluation of ketamine in a Phase 2 clinical study in patients suffering from ALS, PharmaTher has a pipeline of products including KETABET™, ketamine for Parkinson’s disease, and a microneedle patch for the delivery of psychedelics.
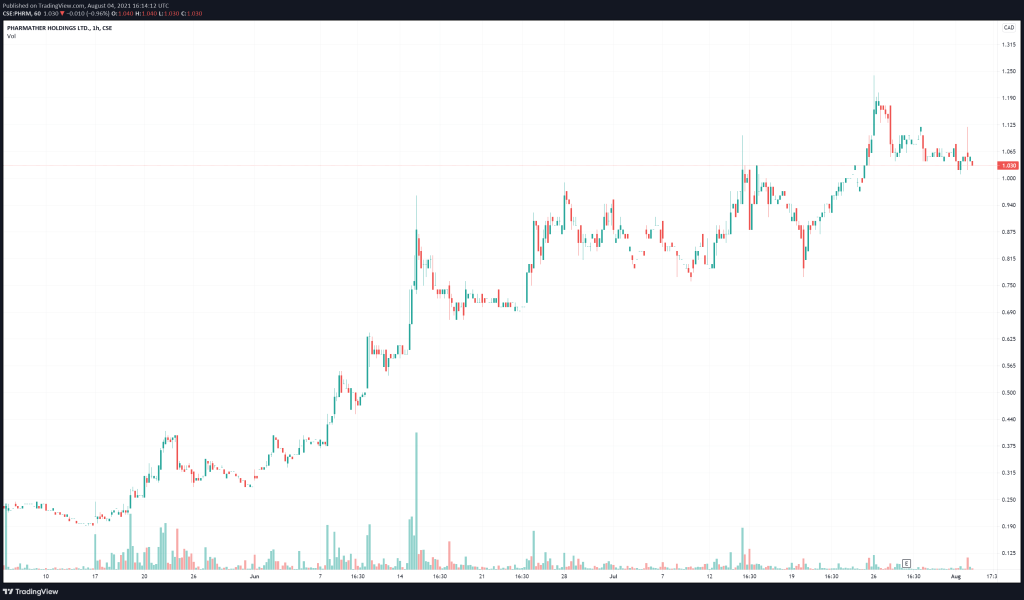
PharmaTher’s share price opened at $1.06, up from a previous close of $1.04. The Company’s shares are up almost 1% and are currently trading at $1.05 as of 11:27 AM ET.