Mydecine Innovations (MYCO.NE) announced they are filing a new patent for their MDMA-like compounds.
In the press release Mydecine writes that “this expansive patent would allow Mydecine to scale its coverage in drug development research in lieu of promising discoveries and FDA-approval progress for novel therapeutics, including granting Breakthrough Therapy Designation to MDMA for the treatment of PTSD in recent years.”
Okay, I have to admit, I have no idea what the fuck they mean by “in lieu of” in that sentence. If my knowledge of unnecessary writing flourishes (and it should, it’s one of my few well-honed skills) and my bilingualism are serving me well, in lieu of just means instead of, but that doesn’t really make any sense here.
Here’s what I think they meant to say: “this expansive patent would allow Mydecine to scale its coverage in drug development research, in step with promising discoveries and FDA-approval progress for novel therapeutics…” but I can’t say for sure. (Let this be a lesson to all the budding PR people out there, as Mark Twain said “Don’t use a five-dollar word when a fifty-cent word will do.”)
Anyways, back to the substance of things: Mydecine is getting into the MDMA game and they filed a patent on entactogenic compounds. They hope through the patent and their associated research to reduce harm and improve the safety of their compound while still providing the same benefits for patients.
MDMA has been getting a lot of love recently, making it’s way to the pages of The New York Times and, as MYCO noted, receiving a breakthrough designation from the FDA gave a breakthrough designation to the Multidisciplinary Association for Psychedelic Studies (MAPS) relating to MDMA. This shows the FDA “has agreed that this treatment may have a meaningful advantage and greater compliance over available medications for PTSD.”
MDMA is hot right now, and so Mydecine is getting involved.

There are some safety concerns with MDMA (more so than psychedelics like psilocybin), and so patenting an MDMA-like compound which has been proven safe could be very lucrative.
“At Mydecine, we are excited to expand our drug development program to include the improvement of entactogenic compounds,” stated Rob Roscow, Chief Scientific Officer for Mydecine. “The groundbreaking progress that we’ve seen from the Multidisciplinary Association for Psychedelic Studies (MAPS) in its Phase 3 clinical trials of MDMA-assisted psychotherapy for Post-Traumatic Stress Disorder (PTSD), including receiving Breakthrough Therapy Designation from the Food and Drug Administration (FDA), shows great promise to date. It is our belief that the tailoring of the properties of these compounds will vastly improve their utility to medicine and therapy. This filing is part of our continued efforts to grow our robust IP portfolio as we consistently file for new patents that offer high potential to expand psychedelics for medical use.”
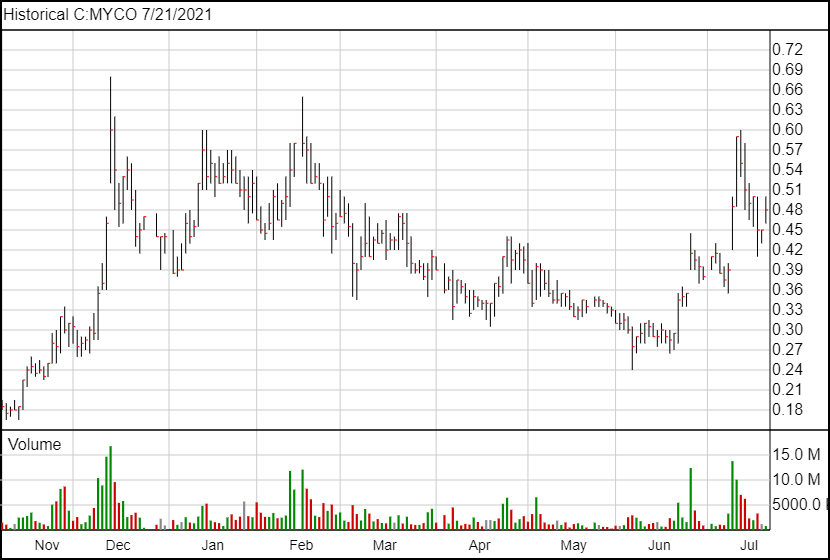
Following the news, MYCO shares are up 3 cents and are currently trading at $0.48.