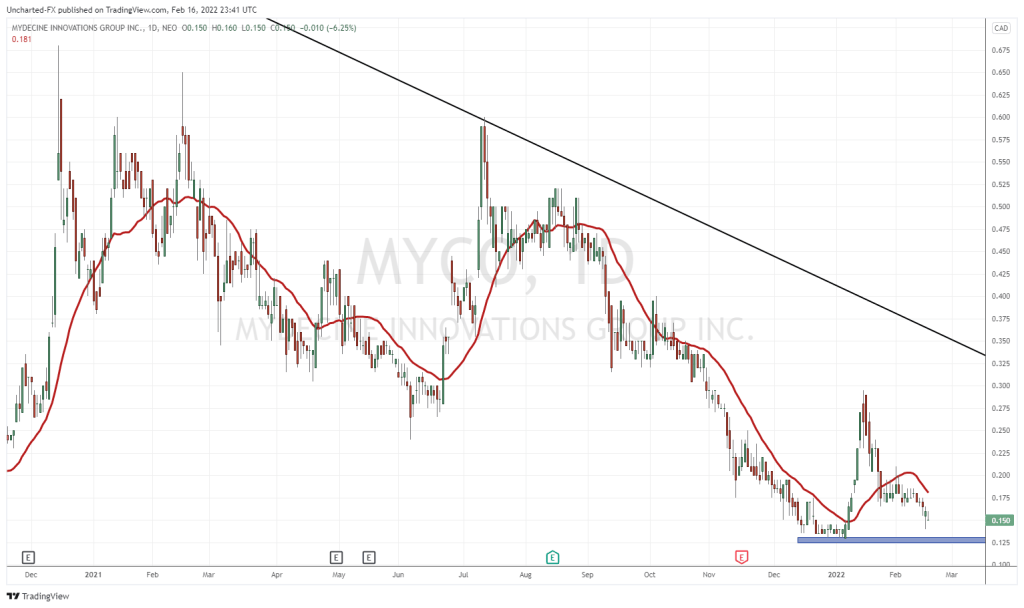
On February 16, 2022 Mydecine Innovations Group (MYCO.NEO) announced the inclusion of a novel molecule with potentially heart-safe microdose enabling properties in their family of psilocin analogs.
Founded in 2020, Mydecine Innovations is a biotechnology company developing therapeutics to treat unmet needs in mental health and addiction disorders.
Focusing on psychedelic compounds with therapeutic potential, Mydecine aims to responsibly fast track the development of novel medicines.
Mental health and addiction disorders are two monsters that are ripping a financial and emotional hole through modern society.
Two years ago, my niece phoned her boyfriend (also the father of her children) and told him she loved him. As she hung up, the boyfriend heard a train in background. The next morning her body was found severed on the tracks.
These grim outcomes are not just symptoms western decadence.
Switzerland, Kazakhstan and Transylvania are dealing with the same issues.
Mydecine’s group of patent-pending molecules is called MYCO-005.
“This family of second-generation molecules directly addresses delivery and stability concerns with the first-generation compounds,” stated MYCO, “Research findings indicate this family of molecules includes a psilocin analog that could potentially be considered a heart-safe microdose drug by eliminating a possible known risk factor.”

“The use of mind-altering mushrooms has pervaded human society since long prior to the birth of civilization,” states Double Blind Magazine.
“Psilocybin has been shown by Functional Magnetic Resonance Imaging (fMRI) to create a state of hyperconnectivity between brain networks, foster an increase in neurogenesis (the creation of brain cells), and drastically alter thought pathways,” the magazine continued.
The jury is still out on the long-term therapeutic benefits of ‘magic mushrooms’ – but a steady flow of positive clinical studies and anecdotal field reports is moving regulatory needle.
“The federal government restored doctors’ ability to request access to psilocybin and MDMA this month after eight years of excluding the non-market prescriptions through Health Canada’s Special Access Program,” reported The Vancouver Sun on January 18, 2022.
“It’s a step in the right direction,” says Dr. Evan Wood, a clinician-scientist from the B.C. Centre for Substance Use.
“There is a growing body of research that shows the efficacy of psychedelic-assisted psychotherapy to treat a broad range of mental health conditions including post-traumatic stress disorder,” reported The Sun.
“A 2020 clinical trial involving 90 adults with chronic PTSD for an average of 14 years, including some at B.C.’s own Centre for Disease Control, found that MDMA-assisted treatment reduced the severity of 88% of the participants’ symptoms”.
Microdosing refers to consuming a very low dose of a psychedelic substance to retain some of the benefits without undergoing a psychoactive experience.
“Although microdosing has been gaining popularity in mainstream media as a possible treatment for indications such as ADHD, depression and anxiety,” stated MYCO, “More research is needed to confirm the safety and efficacy of this method.
“When you consider any drug intended to be taken over a long period of time, there is often a strong medical risk involved,” said Mydecine Chief Scientific Officer Rob Roscow. “When it comes to psilocybin in particular, there are cardiovascular health concerns due to the binding affinity to the 5-HT2B receptor.”
Previous studies have indicated that consuming low doses of psilocybin over a long period of time could cause certain cardiac risks.
“Through our ongoing research, we have found that one of our psilocin analogs is showing strong binding at the classic psychedelic 5-HT2A receptor, but is not binding to the 5-HT2B receptor,” stated Roscow, “This is a strong indication that our improved psilocin analog could potentially produce the same benefits of natural psilocybin with an increased safety profile for microdosing.”
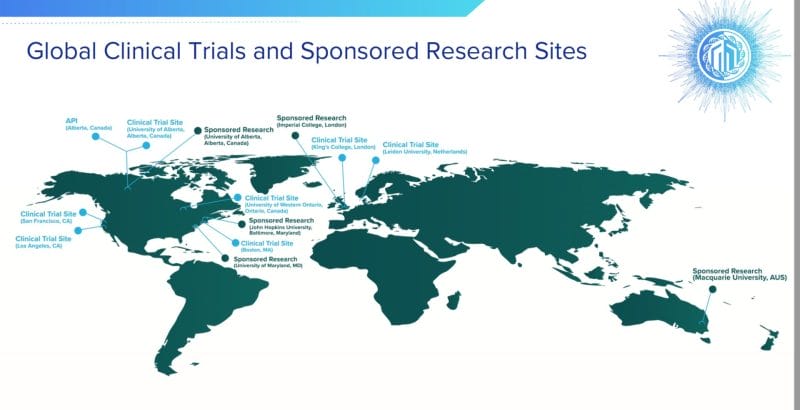
On February 1, 2022 Mydecine announced that it has submitted a pre-IND briefing package to the U.S. Food and Drug Administration (FDA) for a clinical study evaluating MYCO-001 in a structured smoking cessation treatment program.
The study will be led by Principal Investigator Dr. Matthew Johnson, Ph.D., Professor of Psychiatry and Behavioral Sciences at Johns Hopkins University
The study will assess the safety and efficacy of psilocybin-assisted psychotherapy utilizing MYCO-001 to treat tobacco addiction.
The scale of this problem is difficult to wrap your head around.
“Tobacco kills more than 480,000 people annually – more than AIDS, alcohol, car accidents, illegal drugs, murders and suicides combined,” reports TFK, “Tobacco costs the U.S. over $225 billion in health care expenditures and more than $180 billion in lost productivity each year.
“From a scientific standpoint, nicotine is just as hard, or harder, to quit than heroin, but people don’t recognize that,” stated Dr. Neil Benowitz, a nicotine researcher at the University of California, San Francisco.
“Tobacco use is the greatest single, preventable cause of death in the world,” stated Bartch, “yet there are few safe and effective treatments for nicotine addiction. As the only company currently investigating a psilocybin compound for smoking cessation, Mydecine is proud to be at the forefront of this research.”
The planned study will build on previous smoking cessation studies conducted by Dr. Johnson and his team at Johns Hopkins University.
In their ongoing trial, 59% of participants who received psychedelic-assisted therapy remained abstinent from smoking at 12 months, compared to only 28% of patients who received the transdermal nicotine patch.
Mydecine’s Phase 2/3 study is projected to launch in Q2 2022 and will be looking at primary endpoints of three and six months.
A combined-phase or operationally seamless clinical trial, such as this one, combines two or more phases into one study and uses results acquired throughout the trial to adjust the course of the study. This design can use resources more efficiently and often can be more informative than a traditional fixed study.
“Even with a wide variety of approved treatments on the market, tobacco addiction continues to remain largely untreated,” added Mydecine CMO Dr. Rakesh Jetly. “With safety and efficacy concerns about current therapies, including the recall of the blockbuster treatment Chantix, there is a strong need for innovative and improved treatment options.”
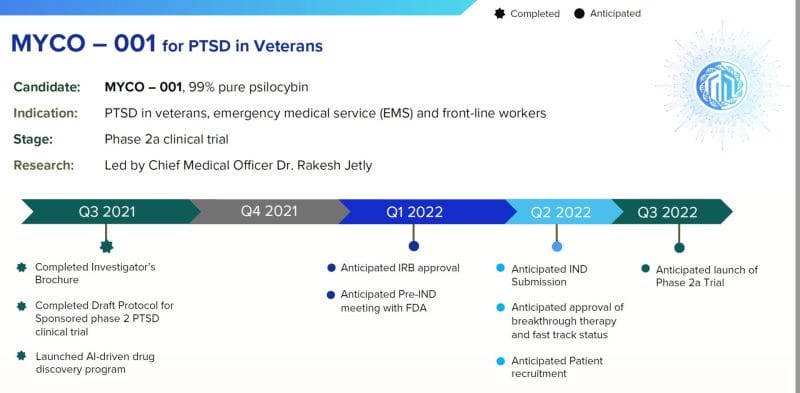
On January 24, 2022 Mydecine announced a partnership with Combat Stress (Combat Stress) and the King’s College London to utilize psilocybin as part of a psychoactive-assisted psychotherapy treatment for post-traumatic stress disorder (PTSD) in veterans.
“During my 31 years as a medical officer and psychiatrist in the Canadian Armed Forces, I have seen first hand the impact PTSD has on our vets and their families,” stated Jetly “Sadly, many soldiers and veterans do not respond to the evidence-based treatments readily available to them and the search is on for new safe and effective treatments.”
“There is a big unmet need when it comes to treating PTSD and other mental health conditions,” stated Bartch, “especially within the veteran population, and this partnership demonstrates the strong progress we continue to make in bringing psychoactive-assisted psychotherapies to the forefront of the market and bring hope for all those struggling with these conditions.”
In this corporate video, some of Mydecine’s founders talk about clinical trials and MYCO’s robust pipeline of drug development.
“We’ve been fortunate enough to progress into a Phase 11 clinical study, utilizing psilocybin assisted psychotherapy for the treatment of PTSD,” stated Bartch, “Every 72 minutes in the United States alone of veteran commit suicide”.
“Medicine innovations group is very passionate about helping people with PTSD, specifically in the veterans’ population. There’s been a complacency in the treatment of PTSD. Currently, there’s not a single pharmaceutical drug that’s manufactured to treat PTSD.
This needs to change. Harnessing nature’s natural design as a starting point, and then applying a pharmaceutical lens to improve upon them to make a treatment more compatible with therapy. That’s exactly what we’re doing here at Mydecine.”

In supervised therapeutic session, Patients are sometimes asked: “How do you want to spend the rest of your time alive?”
“What we’re seeing is patients’ default thinking brain steps aside to reveal their unconscious,” stated Dave Phillips, a registered clinical counsellor, “People are able to resolve decades-old trauma and fear and are seeing long-lasting gains during psychedelic-assisted treatment.”
For Abbotsford patient Laurie Brooks, who is battling a terminal cancer diagnosis, the answer dawned upon her after her first session with Phillips after a legal dose of psilocybin she got through a Sec. 56 exemption.
“For me, the mushrooms took the anxiety out of cancer. It changed my perspective to see that the disease is not my entire life,” the 53-year-old said.
“We are very excited about our MYCO-005 family of molecules,” stated CEO Josh Bartch in the February 16, 2022 PR, “Not only have we made improvements to this second generation of compounds to specifically address concerns for medical use, like onset time and shelf stability, but now we believe we have also identified a microdosing compound that is safer than what’s currently available on the market.
Full Disclosure: Mydecine is an Equity Guru marketing client.