Bionano Genomics (BNGO.Q) has released a summary of results obtained by the University Hospitals Leuven, Belgium utilizing Saphyr® System, the Company’s Optical Genome Mapping (OGM) platform.
The work completed by Dr. Dewaele and her team in developing an OGM assay for ALL subjects and comparing it to traditional methods has generated a dataset that makes a compelling case for the value of using OGM with our Saphyr system,” said Erik Holmlin, PhD, CEO of Bionano Genomics.
The study conducted by the University Hospitals Leuven developed an assay for whole genome analysis of acute lymphoblastic leukemia (ALL) subjects. ALL refers to a type of cancer that starts in the bone marrow, the spongy tissue inside some bones that is responsible for producing red blood cells. However, ALL interferes with blood cell generation, resulting in the production of immature red blood cells. This can result in a wide range of symptoms including fatigue, frequent bleeding, bruises and joint pain.
With this in mind, there are a variety of ways to diagnose ALL, including karyotyping. In particular, fluorescence in-situ hybridization (FISH) testing is commonly used to detect chromosomal abnormalities, however, a study conducted by the Journal of Pediatric Hematology/Oncology suggests that FISH should only be used as a complementary diagnostic. According to the study, diagnostic accuracy of FISH for detecting various abnormalities related to ALL were unreliable. While FISH demonstrated high accuracy in detecting some prognostic indicators, it was unable to detect others.
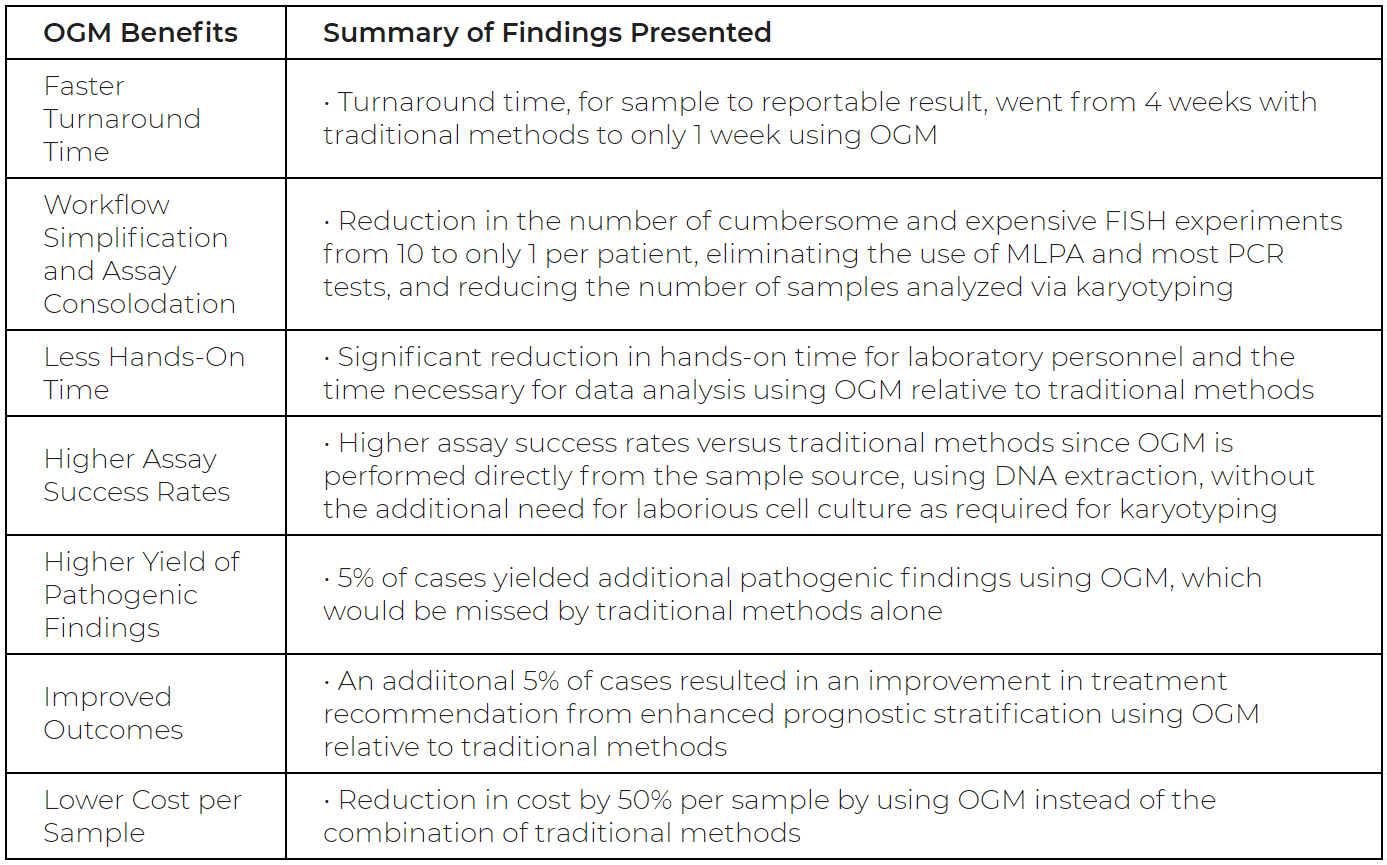
In contrast, genome analysis utilizing Bionano’s Saphyr® System resulted in a workflow with significantly faster turnaround time, higher success rates, and lower cost per sample compared to traditional ALL diagnostics like FISH. In a study of 40 ALL subjects whose samples were analyzed by the Saphyr® System, Bionano’s OGM showed 100% concordance compared to traditional methods with no false positives. A full chart outlining the study results can be seen below.
“They have implemented this assay and it has been evaluated and accredited as part of a novel workflow that transforms the way genome analysis for ALL happens in their institution and paves the way for new assays, including one developed for FSHD,” continued Erik Holmlin.
According to Bionano’s Q1 2021 financial results, the Company’s cash and cash equivalents are sitting at $362,057,000, growing substantially from $38,449,000 in the previous quarter. Bionano’s assets increased to $384,861,000 and its liabilities shrank to $22,143,000 in the same period, resulting in total stockholders’ equity of $362,718,000. Dr. Barbara Dewaele, who oversaw the recent study, summarized that their hospital plans to develop more OGM-based assays for other types of leukemias in the future. With this in mind, they have already developed one for muscle disease facioscapulohumeral musical dystrophy FSHD).
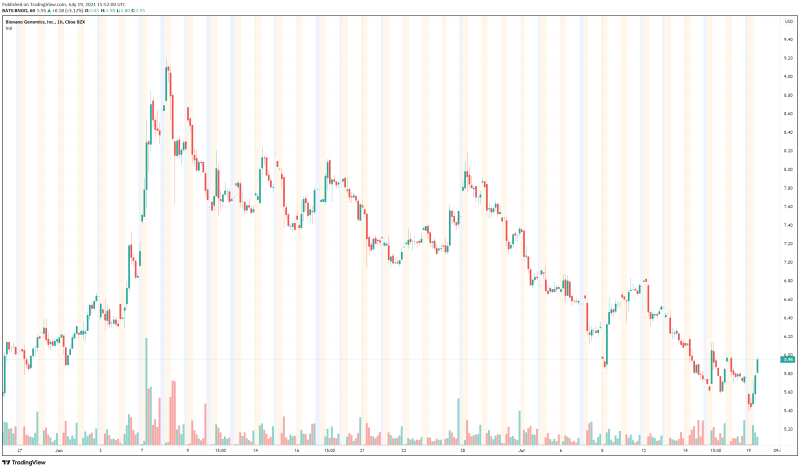
Bionano’s share price opened at $5.61, down from a previous close of $5.77. The Company’s shares are now up 3.21% and are currently trading at $5.97 as of 11:53AM ET. This indicates that there has been noticeable change following the news.