Bionano Genomics (BNGO.Q) announced today that it has made significant progress in China with the adoption of its Saphyr System for optical genome mapping (OGM) by WeHealth Shanghai, a leading provider of genome analysis services in reproductive health.
“This week’s WeHealth symposium demonstrates the success of our market development efforts in China…We are seeing both a growth in Saphyr adoption and an expansion of its applications. The China market is important in part because it represents one of the world’s largest in reproductive health and genetics. We continue to invest in our commercial operations in China, driven by our new commercial leaders, CCO Jason Priar and China business leader Li Yu. We look forward to seeing continued progress in China as a result of these efforts,” commented Erik Holmlin, PhD, CEO of Bionano Genomics.
The adoption of Bionano’s Saphyr System was announced at the Structural Variation Symposium (SVS) in Shanghai, organized with support from the Shanghai Society of Genetics. WeHealth announced the launch of its complete genome-analysis offering using the Company’s OGM combined with whole-exome or whole-genome sequencing. In addition to WeHealth’s announcement, the SVS also featured a series of scientific presentations covering a wide range of OGM applications in genome analysis, including reproductive health. To put things into perspective, lets go over three of the most noteworthy presentations and findings.
During one presentation, Dr. Xiangdong Kong from the First Affiliated Hospital of Zhengzhou University disclosed his findings using OGM for prenatal testing in families with a history of facioscapulohumeral muscular dystrophy (FSHD). FSHD refers to a genetic muscle disorder most commonly affecting the muscles of the face, shoulder blades, and upper arms. According to the Muscle Dystrophy Association, FSHD is the third most common type of muscular dystrophy with an estimated prevalence of about 4 cases per 100,000 individuals. Since the current standard of care for FSHD analysis uses outdated technology requiring quantities of DNA far greater than can be collected via aminocentesis, prenatal testing for FSHD has not occurred in China.
However, using OGM, Dr. Kong was able to successfully analyze 12 prenatal specimens for FSHD, representing an opportunity to update China’s antiquated standard of care regarding FSHD. Next, Dr. Miao Jiang from The First Affiliated Hospital of Soochow University showed that OGM can replace four traditional techniques with a single assay using OGM in testing for hemophilia A, a blood clotting disorder.
Last but not least, Dr. Chenming Xu from the Obstetrics & Gynecology Hospital of Fudan University used OGM to analyze genomes of parents suffering from recurrent pregnancy loss (RPL). OGM was able to successfully identify structural variants in the parents at sufficiently high resolution, bypassing the need for fluorescent in-situ hybridization (FISH) when selecting embryos. In comparison to OGM, FISH is considerably more expensive and slower. With this in mind, OMG was able to demonstrate its potential as an alternative for preimplantation genetic testing.
That’s enough scientific mumbo-jumbo for one article. The bottom line is, OGM has proven itself as a versatile, innovative and effective tool. Having now been adopted by WeHealth, Bionano’s Saphyr System has provided the Company with a foot in China’s expansive reproductive health and genetics market. Overall, Bionano has performed quite well recently, achieving a 179% year-over-year revenue increase in Q1 2021. With this in mind, Bionano may be worth keeping an eye on.
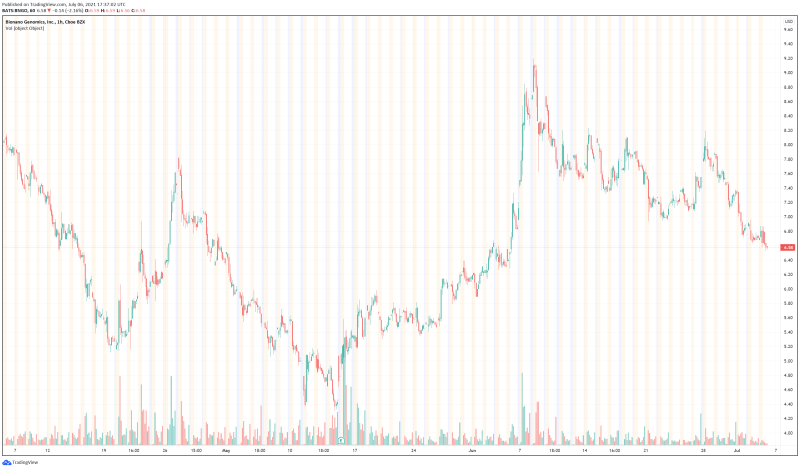
Bionano’s share price opened at $6.81, up from a previous close of $6.72. The Company’s shares are down -2.08% and are currently trading at $6.59 as of 1:40PM ET.