Field Trip Health (FTRP.T) announced that it has completed initial drug metabolism and pharmacokinetic (DMPK) studies for FT-104.
Field Trip completed in vivo and in vitro studies. The in vitro studies showed that metabolization of FT-104 is rapid and complete, converting FT-104 into the active form of the drug with relatively few metabolites.
Field Trip’s in vivo pharmacokinetic studies confirmed a “fast, efficient, and complete conversion of FT-104 to the active, plus a fast elimination of both prodrug and active compounds from the systemic circulation” according to their press release.
From the combination of the in vivo and in vitro models, Field Trip believes they can use well-designed preclinical toxicology methods to address the pharmacology and safety of FT-104.
From the combination of the in vivo and in vitro models, Field Trip believes they can use well-designed preclinical toxicology methods to address the pharmacology and safety of FT-104. They also believe that they have identified what they need to do to ensure an overall trip time of less than 4 hours, something which makes the drug easier to study in a research setting and easier to https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngister in a therapeutic setting.
“From the inception of our drug development efforts, we set out to develop a novel, next-generation psychedelic molecule that would be easy to synthesize, had low risk for addiction, that offered similar potency and experience to psilocybin, but with a much shorter duration to make it more clinically efficient and accessible,” stated Dr. Nathan Bryson, Field Trip’s Chief Science Officer and the inventor of FT-104. “The completion of our DMPK studies confirms that FT-104 meets all of those criteria, and with synthesis and scale-up now complete, we are getting the pieces in place to submit our applications to commence Phase 1 human trials.”
FT-104 is a novel, synthetic psychedelic molecule developed by Field Trip, which was inspired by the chemical structures of known psychedelic substances. FT-104 was selected for its simplicity as well as its potential to distinguish itself with respect to its pharmacological features relative to naturally-derived substances, such as psilocybin and DMT, or known lab-created substances like LSD.
FT-104 is a novel, synthetic psychedelic molecule developed by Field Trip, which was inspired by the chemical structures of known psychedelic substances.
Field Trip also announced that they have secured a Good Manufacturing Practices (cGMP) compliant contract manufacturer to produce FT-104. FTRP began synthesis optimization and scale-up in the fourth quarter of 2020 and has produced its first-kilogram scale engineering batch of FT-104. This production of FT-104 will help Field Trip complete its formulation and pre-clinical development, and another batch will be produced for the Phase I trials.
Field Trip has been on an upward trend since being listed on the Toronto Stock Exchange, having gone up more than 25% in the two weeks since the listing. Field Trip also recently applied to join the Nasdaq.
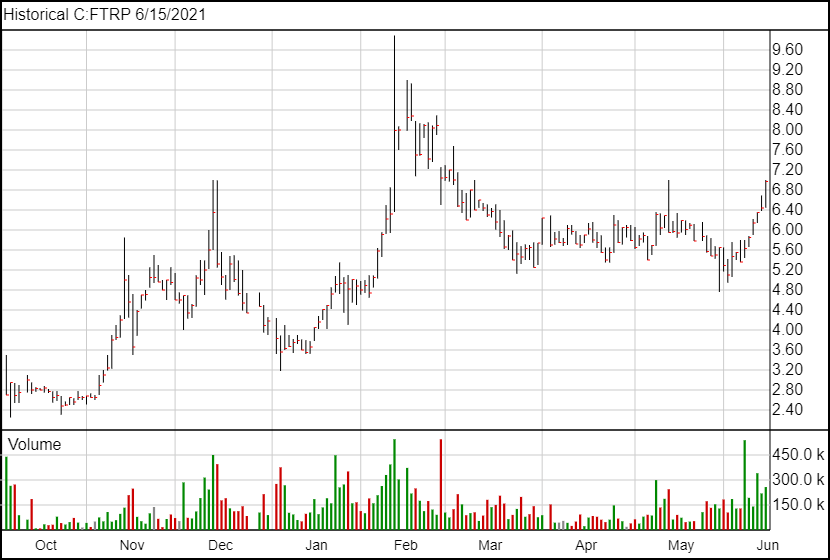
Following today’s news, Field Trip is up 50 cents and is currently trading at $6.94.