Tetra Bio-Pharma (TBP.T) has announced the start of the REBORN1© clinical trial, designed to evaluate the effect of QIXLEEF™, the Company’s inhaled proprietary drug formulation. During this study, QIXLEEF™ will be compared to immediate release oral morphine sulfate, which is conventionally used to provide onset pain relief in people living with cancer.
“Today we recognize an important milestone in advancing the clinical development of this new potential therapeutic for people living with cancer pain. Cancer pain is usually managed with a strong opioid. We believe QIXLEEF™, if proven to be safe and effective, would provide patients with cancer pain a safer treatment option with potentially greater benefits than the current standard of care. QIXLEEF™ may in fact transform the pain market, an area that has been stagnant for many years…,” commented Dr. Guy Chamberland, CEO and CRO of Tetra.
Opioids are responsible for a majority of overdose deaths in the U.S. today, yet opioids are still largely prescribed for indications in pain relief. Opioids trigger the release of endorphins, the feel-good neurotransmitters in our brains, which numb feelings of pain while increasing pleasure. As a result, opioids are highly addictive and indiscriminate. While personal history and the length of time used do have an impact, its generally impossible to predict who’s vulnerable to opioid addiction.
With this in mind, Tetra Bio-Pharma’s QIXLEEF™ is a botanical drug product with a fixed ratio of THC and CBD, inhaled through a Class 2 vaporizer, a medical device approved by Health Canada. The REBORN1© study is being conducted in the United States in collaboration with with Hassman Research Institute, a clinical research organization. Twenty adults living with breakthrough cancer pain (BTcP) who are currently receiving stable opioid treatment, will be enrolled in the study to assess whether Tetra Bio-Pharma’s inhaled QIXLEEF™ is able to control BTcP faster than immediate-release morphine sulfate tablets. Keep in mind, morphine is one of many drugs classified as an opioid.
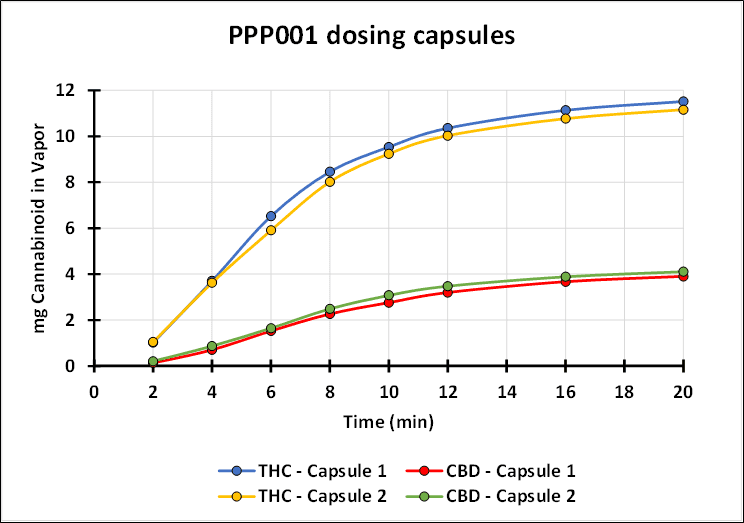
“Tetra has spent years studying the inhalation of cannabinoids from both synthetic and botanical sources. This research has shown that our investigational new drug, when used with a proprietary medical device, can deliver to patients a reproducible and consistent profile of cannabinoids. The graph below demonstrates the consistency of the inhaled delivery of THC and CBD. We confirm that QIXLEEF™ has arrived in the United States and the trial activities are set to begin,” continues Dr. Guy Chamberland.
The REBORN1© clinical trial will take place over the course of ten weeks. Tetra Bio-Pharma is hopeful that QIXLEEF™ will prove itself as a safe, reliable, and effective alternative to orally https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered morphine sulfate tablets.
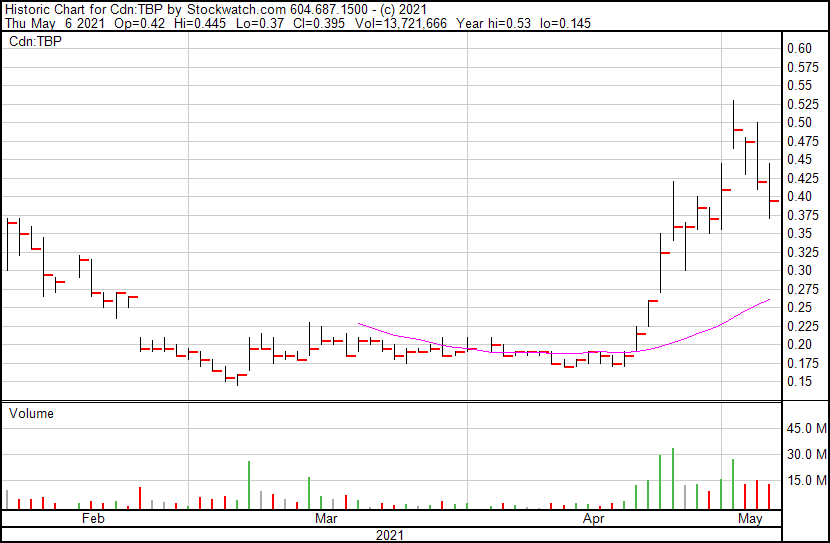
Tetra Bio-Pharma’s share price opened at $0.42 and is currently trading at $0.40 as of 12:53PM ET. The Company’s share price is currently down 2 cents following the news.