Another day, another Tetra Bio-Pharma (TBP.T) news release. A day after they were granted a Drug Establishment Licence by Health Canada for their REDUVO soft gel capsules, Tetra announced they received a one-year extension on their veterinary ophthalmic drug, PPP-003v, in the treatment of indolent corneal ulcers in companion animals.
The end of the trial’s enrolment had been scheduled for April 30, 2020, but concerns about the spread of COVID-19 caused them to halt the trial. Tetra hopes to show that PPP-003v can treat indolent corneal ulcers which occur in dogs, especially Boxers and Corgis, causing them pain and inflammation.
Current treatment methods for indolent corneal ulcers involve minor surgery (which requires anesthesia) and injections, and success rates are estimated at ~90% with an average recovery time of two weeks. Tetra is developing a treatment for the inflammation and pain that comes with these ulcers that contains a synthetic Cannabinoid 2 receptor selective agent. You know how people take cannabis for their glaucoma? This is sort of like that, but for your dog.

“We are pleased with this regulatory authorization and the ability to re-activate the trial. While the active pharmaceutical ingredient used in the PPP-003v drug formulation is the same as the one used in ARDS-003, Tetra’s innovative immunomodulator drug concurrently being developed for COVID-19, there is a major difference with how the drug is delivered. PPP-003v is intended to be used as a topical medication and is delivered as a sterile eye drop and ointment, while ARDS-003 is a sterile injectable nano-emulsion finished drug product,” said Dr. Guy Chamberland, CEO and CRO of Tetra Bio-Pharma.
Tetra claims this study will be the first time a synthetic cannabinoid agent is going to be used in a clinical setting for companion animals to treat ophthalmic pain and inflammation. (Of course, as VICE has documented, it’s not the first time outside clinical settings.) Based on positive initial proof-of-concept clinical trial results, Tetra will submit a new clinical trial application with the Veterinary Drugs Directorate of Health Canada and commence filing for New Animal Drug Application with the FDA for PPP-003v.
With pets “going like hot cakes” during the COVID pandemic, many expect the veterinary health market to grow rapidly in the upcoming years. In 2020, the pet eye care market was $148.8 million USD, and it is expected to grow at a CAGR of 5.7% over the next 5 years.
“The PPP-003 program, including PPP-003v, represents a significant opportunity for Tetra since there is a substantial unmet medical need for painful inflammatory eye disease8. Tetra’s veterinary ocular program is a key component of this program and the ongoing pilot phase trial will provide important data which can potentially be included as part of the pre-clinical data that will be submitted to support PPP-003 use in human clinical trials,” commented Dr. Guy Chamberland.
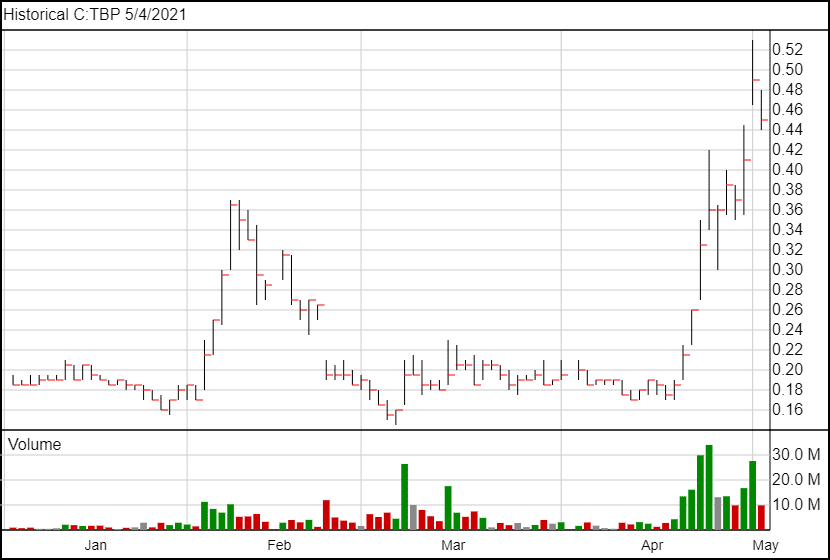
Following the news, TBP’s share price fell 4 cents, and it is currently trading at $0.45.