Numinus Wellness (NUMI.V) partnered with KGK Science, a contract research organization focusing on natural products, for a few psilocybin extraction clinical trial today, according to a press release.
The Phase 1 trial will include 14 human volunteers, and the trial’s purpose will be to determine the safety and psychoactive properties of a Psilocybe mushroom product, extracted by Numinus Bioscience at their British Columbia based laboratory. If the company successfully completes the trial, they’re hoping they will have enough safety and efficacy data for a psilocybin product usable for future research, as well as a government-approved potential special access or compassionate access designation.
“This Phase 1 trial supports our mandate to improve access to safe, evidence-based psychedelic-assisted therapies in Canada and around the world. Through this trial, we expect to demonstrate that Numinus is well ahead in the development of natural Psilocybin therapeutics that are safe for humans and can be used to support our sector’s growth through sales to other research organizations, the creation of new intellectual property, and the ability to conduct our own Phase 2 and Phase 3 clinical trials, if we choose to do so,” said Payton Nyquvest, CEO of Numinus.
The raison d’etre for the nascent psychedelics industry is in finding innovative health care solutions to pressing psychological problems like depression, end of life anxiety and substance use disorders. The Numinus Wellness model includes psychedelic production, research and clinic care, and they’re a part of the integration of psychedelic assisted therapies into mainstream clinical practice.
Numinus is only three years old as a company, and has already completed the first legal extraction of Psilocybe mushrooms in Canada by a private company for research purposes. They’ve also finished their first round of cultivation and harvesting of the first legal Psilocybe mushrooms.
“We are excited to see Health Canada grant legal exemptions for certain patients to use Psilocybin alongside psychotherapy in situations when other treatments have failed, and to consider revisions to its Special Access Programme that could allow Canadian patients access to psychedelic medications when other drugs have been unsuccessful. As research interest and the potential for increased compassionate access to Psilocybin grows, it is imperative that patients have access to safe, pharmaceutical-grade supply that offers consistent psychoactive properties at scale. With this trial, we are taking the first steps towards that goal,” said Dr. Evan Wood, chief medical officer of Numinus.
In addition to KGK Science, Numinus is also partnering with global contract researcher, Syreon Corporation, for a single-arm, open-label compassionate access trial to determine the efficacy and safety of psilocybin-assisted motivational enhancement therapy using synthetic psilocybin. The trial will include enrolment of 30 individuals with opioid, stimulant and/or alcohol use disorders, and be held in Vancouver, and add to the growing body of research.
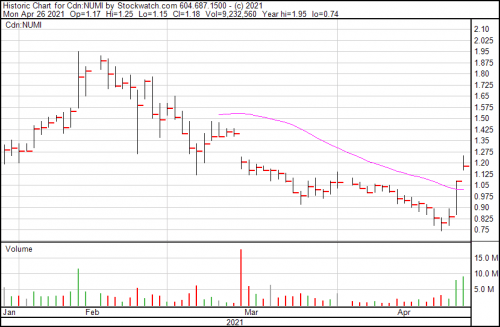
Numinus Wellness is up $0.10 so far today, and is now trading at $1.18.
—Joseph Morton