Cybin (CYBN.NE) announced its plans to advance the pre-clinical work for two of its molecules: its orally dissolving tablet (ODT) formulation CYB003 and its inhaled formulation CYB004.
These pre-clinical studies are required by the US Food and Drug Administration (FDA) for investigational new drug (IND) applications.
Upon successful completion, the results from the pre-clinical IND-enabling studies will be included in applications to health authorities, such as the FDA, Health Canada, and the European Medical Association.
The two molecules would then advance to Phase I human clinical trials for specific psychiatric conditions. Psychedelic therapy is believed to be effective in dealing with treatment-resistant depression, drug addiction, and various forms of anxiety.
Cybin has enlisted Labcorp Drug Development as their pre-clinical research organization.
“Starting the IND-enabling trials for CYB003 and CYB004 is an exciting and important step forward for Cybin as we progress the study of these molecules. Our scientific team is eager to produce a robust submission to the FDA that will advance our path forward to clinical trials. Once the studies have been completed, we plan to file IND applications, targeting treatment-resistant psychiatric disorders and certain forms of addiction, in 2021,” stated Doug Drysdale, CEO of Cybin.
CYB003 and CYB004 emerged as Cybin’s leading molecule candidates after 20 in vitro and in vivo pre-clinical tests. CYB003 uses Catalent’s proprietary FDA approved Zydis ODT technology, which gives the https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistrator more control over the dosage and the movement in and out of the patient’s body.
Cybin has designed the molecules to have faster onsets and shorter times of duration, which will help in both clinical trials and therapeutic settings. A typical psilocybin ‘trip’, with naturally found mushrooms, can last up to 12 hours. For scientific purposes, this can be a challenge because scientists tend to work in 8-hour shifts, which means a relief team would have to come in and take over partway through. It is also important for therapeutic settings, as therapists may find 12-hour sessions too long.
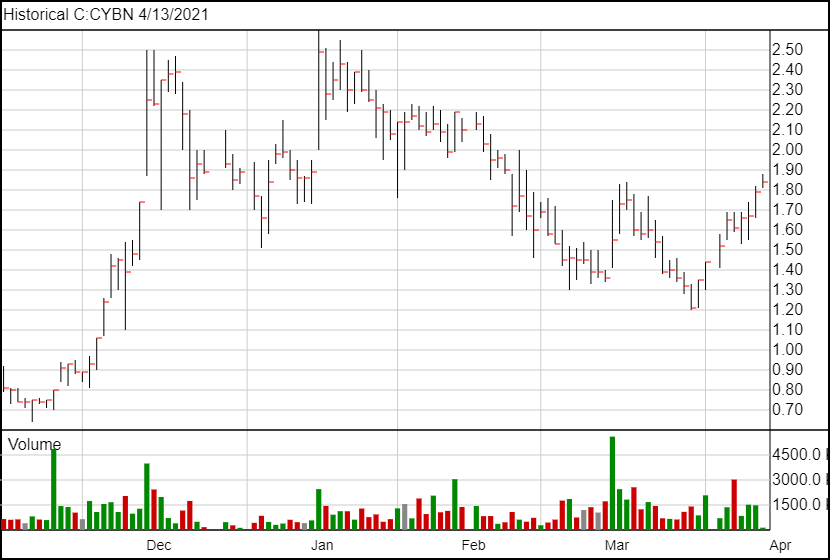
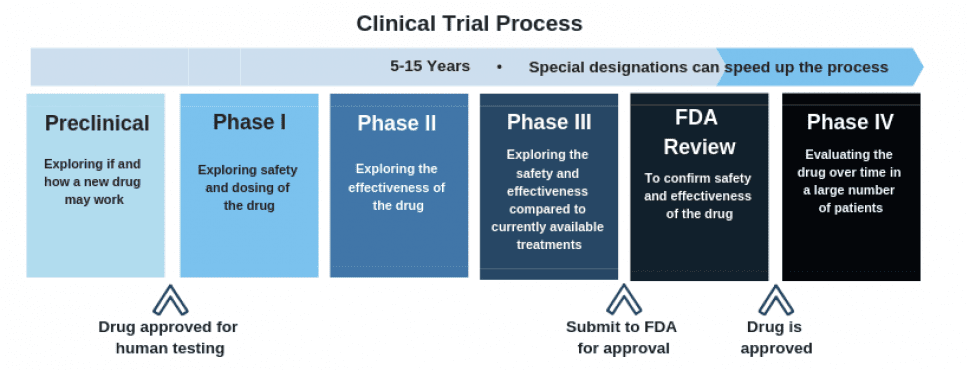 As for the clinical trials, as the chart above shows, this is still early days. Cybin is finishing up the first stage in preparation for their application to move to the next phase.
As for the clinical trials, as the chart above shows, this is still early days. Cybin is finishing up the first stage in preparation for their application to move to the next phase.
Following the news, CYBN is up 5 cents to $1.84 a share.
Full disclosure: Cybin is an Equity Guru marketing client.