Cybin (CYBN.NE) announced they have entered a drug development agreement with Catalent (NYSE: CTLT).
Catalent is a global provider for advanced delivery technologies, development, and manufacturing solutions for drugs, biologics, cell and gene therapies, and consumer health products. Catalent has supported partners in the introduction of 200 new products and the manufacturing of over 70 billion doses each year.
Cybin will be using Catalent’s proprietary Zydis orally disintegrating tablet (ODT) technology for the delivery of their novel deuterated tryptamine (CYB003).
Zydis is a unique ODT that creates a freeze-dried tablet that dissolves instantly in the patient’s mouth without the need for water, which can assist healthcare providers with patients who have trouble swallowing.
CYB003 recently underwent successful pre-clinical testing and has entered full IND-enabling studies in preparation for future clinical testing. CYB003 is being researched as a potential therapy for treatment-resistant psychiatric disorders and helped lab mice recover cognitive function after mild traumatic brain injuries.
“We are excited to partner with the team at Catalent with the aim of developing fast-acting, shorter-duration formulations of CYB003, recently acquired as part of our acquisition of Adelia Therapeutics. Our focus on reducing the need for health system resources, such as in-clinic therapist time, is an important part of our goal to create scalable, more accessible treatments for mental health disorders,” stated Doug Drysdale, CEO of Cybin.
Cybin is a biotechnology company focused on progressing psychedelic therapeutics by improving delivery methods and conducting research on psychedelic therapeutic’s efficacy. They believe psychedelic therapies offer a novel approach to mental healthcare, which focuses on treating the underlying conditions and improving the patient’s experience and outcome, rather than merely treating symptoms.
CYBN believes that using Zydis to deliver CYB003 could have significant benefits. They believe this ODT would allow pre-gastric delivery and prevent first pass metabolism (a phenomenon where the concentration of a drug is reduced significantly by the time it enters the patient’s circulation). These innovations could potentially improve the pharmacokinetic profile of the drug, meaning it would give researchers more control of the movement of the drug in and out of the patient’s body.
Cybin expects the study to begin in April 2021 and will begin with initial feasibility studies for the manufacturing and analytical testing of ODT doses containing varying quantities of CYB003.
Jonathan Arnold, President of Oral and Specialty Delivery at Catalent, commented, “We look forward to working with Cybin to potentially develop a novel and fast-acting therapy for treatment-resistant psychiatric disorders. The Zydis platform is an ideal technology to leverage for this type of drug formulation, as pre-gastric absorption is crucial for efficacy.”
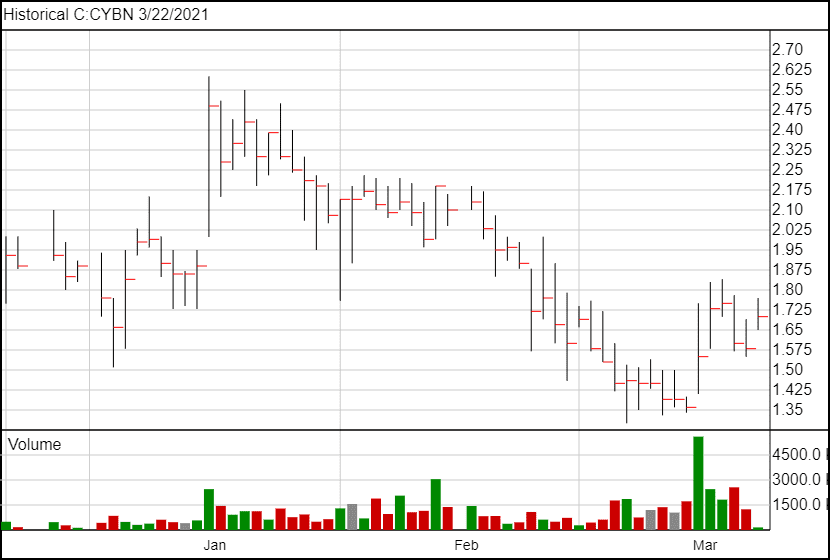
The stock price has risen to $1.67 following the news.
Full disclosure: Cybin is an Equity.Guru marketing client.