Revive Therapeutics (RVV.C) provided an update on their Phase 3 clinical trial for bucillamine for COVID-19 today.
Primarily, the update’s about funding and more specifically their recent $23 million financing. The company intends to use the monies to fund their aggressive expansion from14 clinical sites to 50 to meet their enrolment requires for the study in Q2 2021.
“With our funding completed, we are adding more clinical sites to meet our enrolment goals and be in a position to meet with the FDA to determine the best path forward for EUA approval. We are committed to achieving our mission in making bucillamine the first orally https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered drug to obtain FDA approval and EUA to treat mild to moderate COVID-19,” said Michael Frank, chief executive officer of Revive.
The study is a randomized, double-blind, placebo-controlled trial with safety and efficacy data analyzed at various intervals and made available to the independent Data and Safety Monitoring Board for review, and further recommendations on whether or not the study should continue, stop, or what changes may be required.
Normally, bucillamine is prescribed to treat rheumatoid arthritis, and has been regularly prescribed in Japan and South Korea for over 30 years.
The trial takes 1,000 patients with COVID-19, randomizes them, and gives them either bucillamine or a placebo for 14 days. The objective of the study is the comparison of the frequency of hospitalization or death in patients with mild to moderate COVID-19 receiving bucillamine with those getting the placebo.
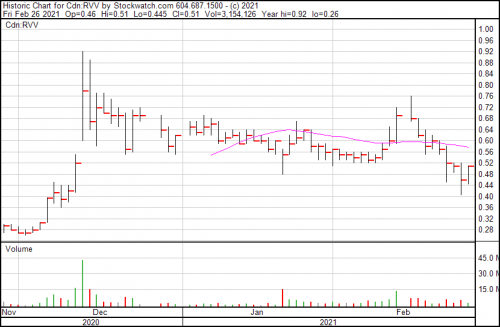
Revive Therapeutics is up 10.1% and closed at $0.51.
—Joseph Morton
Full disclosure: Revive Therapeutics is an equity guru marketing client.