Medipharm Labs Inc, a subsidiary of Medipharm Labs (LABS.T), kicked off a clinical trial to determine the effectiveness of Medipharm Labs’ medical cannabis products and formulations on treating end-stage renal disease or chronic kidney disease (CKD), which is a leading cause of death worldwide.
Medipharm has signed into a Mater Clinical Studies Agreement with Canadian-based, OTT Healthcare. OTT will study the pharmacokinetic (dosing) and safety profile of various cannabinoid formulations for kidney disease patients and assess the effects on their pain and quality of life scores.
“This Agreement is very meaningful for Medipharm Labs as it signals our dedication, as a specialized pharmaceutical company, to sponsoring and directly participating in the intense and ongoing scientific research necessary to bring the benefits of medical cannabis to those in dire need worldwide. The particular area of focus for this very significant Agreement is vitally important. Renal disease is the 11th leading and 6th fastest-growing cause of death globally and those with it suffer from pain, pruritus (severe skin itch), impaired sleep, depression and fatigue. We believe deep research into the use of medical cannabis to treat the effects of this devastating condition is long overdue,” said Pat McCutcheon, CEO of Medipharm Labs.
Data gathered from the trials will be used to support follow-up randomized double-blind clinical trials designed to establish the safety and efficacy of their products. The results, including any new proprietary intellectual property, will be the property of Medipharm Labs.
“Medipharm Labs has established itself as a producer of pharmaceutical quality cannabis API and formulations. With our capabilities and research and development team, we are well positioned to support these trials and provide novel formulations including products from our portfolio and in general to expand our contribution to Nephrology. In broader terms, initiating this clinical trial demonstrates our growing maturity as a research-driven pharmaceutically focused company with global aspirations,” said McCutcheon.
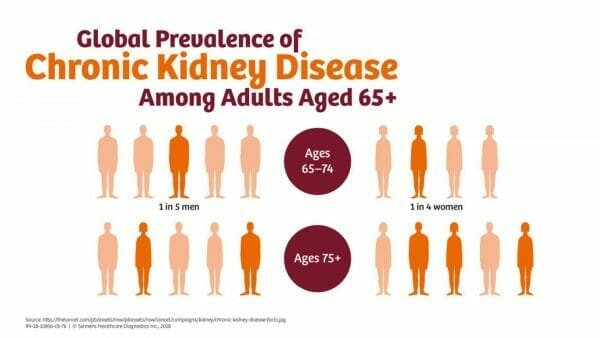
The physical and psychological symptom burden in patients with advanced CKD is significantly debilitating, and it often receives inadequate treatment, according to the Canadian Journal of Kidney Health and Disease. Patients with CKD have a reduced life expectancy, with the average patient living eight to four and a half years after dialysis initiation for those aged 40-64 years.
Therefore, it makes sense that optimization of quality of life would be of the highest priority. Patients in this position may look to medical cannabis to manage their symptoms, including those found in advanced stages of CKD.
—Joseph Morton