On August 31, 2020 Revive Therapeutics (RVV.C) announced that its phase 3 clinical trial protocol to evaluate Bucillamine in patients with mild-moderate COVID-19 has received approval from the independent Institutional Review Board (IRB) at Advarra, a premier IRB services company in North America.
RVV is a specialty life sciences company focused on the R&D of therapeutics for medical needs and rare disorders.
An IRB is an FDA registered constituted group that has been formally designated to review and monitor biomedical research involving human subjects.
An IRB has the authority to approve, require modifications to, or disapprove research.
IRBs use a group process to review research protocols and related materials to ensure the protection of the rights and welfare of human subjects of research.
Bucillamine is a medical Swiss-Army-Knife with an array of utilities including “regulating B-cell function” “thiol antioxidant” and “cisplatin-induced otoxicity”.
“Revive Therapeutics is exploring the use of the drug Bucillamine as a potential novel treatment for infectious diseases including influenza and the coronavirus disease (Covid-19),” reported Equity Guru’s Chris Parry on April 23, 2020.
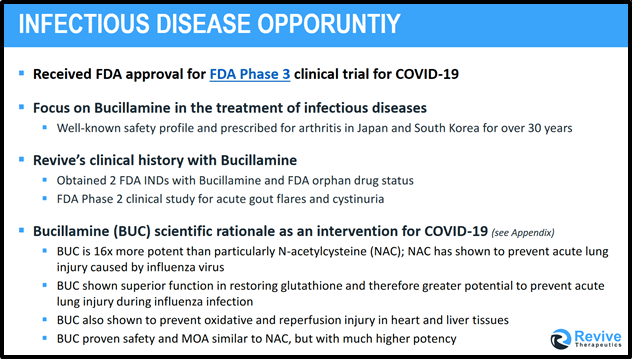
“Bucillamine has potential to attenuate or prevent damage during myocardial infarction, cardiac surgery and organ transplantation,” states the University of Colorado Health Sciences Center, it “enters the cells by the same mechanism that normally transports the amino acid cysteine”.
Preclinical and clinical studies have demonstrated that Bucillamine can significantly reduce the negative symptoms of respiratory viral infections in animals and humans.
The basis of the clinical study will analyze if Bucillamine has the potential, via increasing glutathione activity and other anti-inflammatory activity, to lessen the destructive consequences of SARS-CoV2 infection in the lungs and attenuate (weaken) the clinical course of COVID-19.
“With the IRB approval of the Phase 3 study protocol for COVID-19, we can recruit U.S. clinical sites efficiently, allowing us to move forward with providing Bucillamine to patients under our IND that was approved by the FDA last month,” stated Michael Frank, Revive’s Chief Executive Officer.
The Phase 3 confirmatory clinical study is titled, “A Multi-Center, Randomized, Double-Blind, Placebo-Controlled Study of Bucillamine in Patients with Mild-Moderate COVID-19”.
“Multi-center” means that the trial is carried out at more than one medical institution; “randomized” means that there will a control group https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered a placebo; “double-blind” means that neither the patients nor the scientists know who is receiving a particular treatment.
Phase 3 confirmatory clinical study highlights:
- Enroll up to 1,000 patients that will be randomized 1:1:1 to receive Bucillamine 100 mg three times a day, Bucillamine 200 mg three times a day, or placebo three times a day for up to 14 days
- Primary objective is to compare the frequency of hospitalization or death in patients with mild-moderate COVID-19 receiving Bucillamine therapy with those receiving placebo.
- Efficacy will be assessed by comparing clinical outcomes (death or hospitalization), using the 8-category NIAID COVID ordinal scale.
- Safety will be assessed by comparing pre-treatment adverse events and treatment-emergent adverse events
An interim analysis will be performed after 210 patients have been treated and followed up for 28 days after randomization. The better performing Bucillamine dose at the interim analysis will be selected and patients will then be randomized 2:1.
In other words, if the patients react better to 200 mg Bucillamine, the 100 mg doses will drop out of the study.
With 24.5 million confirmed cases of coronavirus globally, and 840,000 dead, the total economic damage caused by the virus is projected to be between $8.1 and $15.8 trillion.
Given the grim human and financial data, it’s no surprise that biotech companies and their shareholders are focussed on developing coronavirus solutions.
In fact, RVV has other irons in the fire.
A good example of RVV’s product pipe-line diversity is its recent acquisition of Psilocin Pharma Corp, that is advancing the development of Psilocybin-based therapeutics in various diseases and disorders.
Revive’s cannabinoid pharmaceutical portfolio focuses on rare inflammatory diseases and the company was granted FDA orphan drug status designation for the use of Cannabidiol (CBD) to treat autoimmune hepatitis (liver disease) and to treat ischemia and reperfusion injury from organ transplantation.
On August 11, 2020 RVV announced that it has received the first set of orally dissolvable thin film strips initially to be used to deliver psilocybin and subsequently additional psychedelic-derived medicines.
Revive has a partnership agreement with the Reed Research Group out of the University of Wisconsin-Madison to evaluate novel formulations of psilocybin.
“The use of mind-altering mushrooms has pervaded human society since long prior to the birth of civilization,” states Double Blind Magazine.
“Psilocybin has been shown by Functional Magnetic Resonance Imaging (fMRI) to create a state of hyperconnectivity between brain networks, foster an increase in neurogenesis (the creation of brain cells), and drastically alter thought pathways,” the magazine continued.
“We see great promise in delivering psychedelic-based medicines to treat various diseases and disorders,” stated Michael Frank, CEO of Revive, “We look forward to unveil the final prototype in the coming weeks.”

For the last 30 years Bucillamine has been prescribed in the treatment of rheumatoid arthritis in Japan and South Korea. It has been shown to be 16 times more potent as a thiol donor in vivo than NAC 6. The drug is non-toxic with high cellular permeability.
Revive has applied for a provisional patent with the U.S. Patent and Trademark Office entitled “Use of Bucillamine in the Treatment of Infectious Diseases” (serial No. 62/991,996).
RVV is not making any express or implied claims that its product has the ability to eliminate or cure COVID-19 (SARS-2 Coronavirus) at this time.
- Lukas Kane
Full Disclosure: Revive Therapeutics is an Equity Guru marketing client