Formerly dismissed-by-many pharmaceutical company Revive Therapeutics (RVV.C) jumped a whopping 68% Friday upon revealing it has been granted approval to move forward with phase 3 clinical trials on the use of its Bucillamine medication as a potential treatment for patients with mild to moderate COVID-19.
The U.S. Food and Drug Administration (FDA) has approved Revive Therapeutics Ltd. to proceed with a randomized, double-blind, placebo-controlled confirmatory phase 3 clinical trial protocol to evaluate the safety and efficacy of Bucillamine in patients with mild-moderate COVID-19.
Translated: RVV has an existing drug approved for other uses, but suspects that drug may be useful in fighting COVID-19. The US FDA has, in turn, helped them bulldoze through the usual bureaucracy and timelines associated with getting to phase 3 trials because of the urgency of the COVID-19 pandemic.
While the drug known as hydroxychloroquine has been discussed ad infinitum in US political and medical circles over suspicions it may help prevent COVID, all studies done to this point have shown it to have a deleterious effect instead. Bucillamine may follow a similar path, in that it’s an existing drug, used to treat rheumatoid arthritis in South Korea and Japan for the past 30 years, and while we know studies on hydroxychloroquine have shown it isn’t useful for COVID – Bucillamine still has the potential to be.
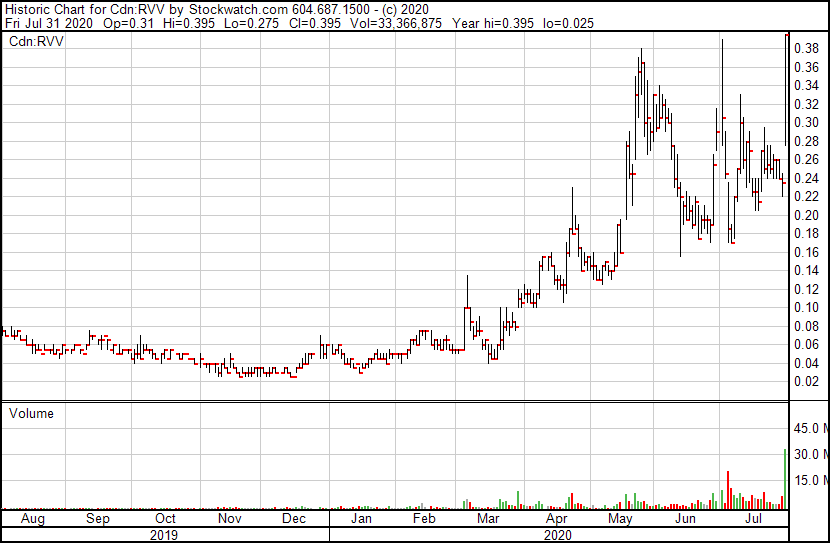
Let’s not get ahead of ourselves just yet – the clinical trials are there for a reason – but interest is growing, especially in Revive’s stock price, which jumped from $0.235 to $0.395 Friday with over 30 million shares traded – good for 13% of the entire float and more than double the previous trade volume record for the stock.
We first mentioned RVV when the stock was at $0.05 in February.
CEO Michael Frank said the approval is, “a tremendous milestone for Revive, and I am very proud of the dedication of our team and partners to bring forward a potential new treatment option for patients with a confirmed diagnosis of COVID-19 globally.”
He added, “We are now focused on executing on our plans for initiating the clinical trial in an expeditious manner.”
Deeper details, for those with the inclination:
The phase 3 confirmatory clinical study, titled, “A Multi-Center, Randomized, Double-Blind, Placebo-Controlled Study of Bucillamine in Patients with Mild-Moderate COVID-19,” will enroll up to 1,000 patients that will be randomized 1:1:1 to receive Bucillamine 100 milligrams three times a day (TID), Bucillamine 200 mg TID or placebo TID for up to 14 days. The primary objective is to compare frequency of hospitalization or death in patients with mild-moderate COVID-19 receiving Bucillamine therapy with those receiving the placebo. The primary end point is the proportion of patients meeting a composite end point of hospitalization or death from the time of first dose through day 28 following randomization. [..] An interim analysis will be performed by an independent data and safety monitoring board (DSMB) after 210 patients have been treated and followed up for a total of 28 days after randomization. The better-performing Bucillamine dose at the interim analysis will be selected, and patients will then be randomized 2:1 to the selected Bucillamine dose or placebo.
I still remember trolls on social media dogging this company and its management team, bleating that they had never made the company work previously and that it was a pump and dump and a ‘fake mushroom deal.’
Michael Frank, take a bow. You’ve more than proved you’re real.
— Chris Parry
FULL DISCLOSURE: Revive Therapeutics is an Equity.Guru marketing client, and we have purchased stock in the company on the open market and through financings.