On May 13, 2020, Revive Therapeutics (RVV.C) provided a corporate update on its pharmaceutical programs for Bucillamine in the treatment of the coronavirus disease (COVID-19) and in the psychedelics area.
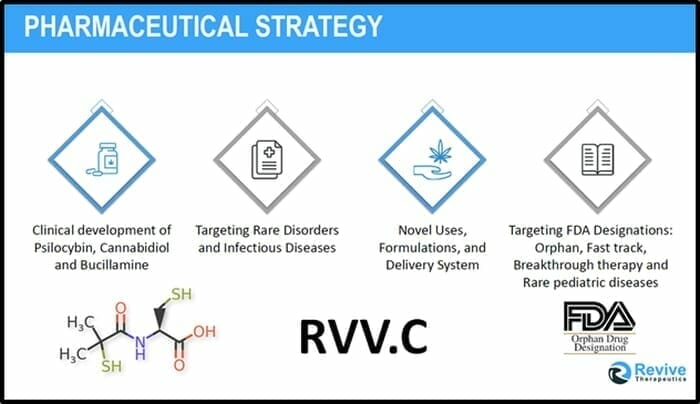
Revive is a life sciences company focused on developing therapeutic solutions for medical needs and rare disorders.
Try Googling “Bucillamine” and you’ll discover that it is a sort of medical Swiss-Army-Knife with an array of utilities including “regulating B-cell function” “thiol antioxidant” and “cisplatin-induced otoxicity”.
“Revive Therapeutics is exploring the use of the drug Bucillamine as a potential novel treatment for infectious diseases including influenza and the coronavirus disease (Covid-19),” reported Equity Guru’s Chris Parry on April 23, 2020.
The company has applied for a provisional patent with the U.S. Patent and Trademark Office entitled “Use of Bucillamine in the Treatment of Infectious Diseases” (serial No. 62/991,996).
“Revive had planned to get approval with the FDA to run phase 2 clinical trials,” continued Parry, “But the FDA had other ideas and has instead PUSHED THEM STRAIGHT TO PHASE 3.
“We are advancing our main programs in COVID-19 and psychedelics with the aim to initiate clinical studies in the short-term while leveraging our assets and building our pharmaceutical-based product pipeline for long-term growth.”
– Revive’s CEO Michael Frank on May 13, 2020
 Bucillamine in the treatment of COVID-19:
Bucillamine in the treatment of COVID-19:
Revive and its CRO Pharm-Olam are preparing the Investigational New Drug (IND) package to give to the FDA for the proposed Phase 3 confirmatory clinical trial to evaluate Bucillamine in the treatment of patients with mild-moderate COVID-19 due to the SARS-CoV-2 infection.
A CRO is a company that provides support to a pharmaceutical or biotechs in the form of research services outsourced on a contract basis.
Pharm-Olam is a 25-year-old company specializing in life-threatening therapeutic areas such as oncology-hematology, infectious disease, and rare disease. It has 25 offices around the world.
The FDA recommended Revive proceed directly into a confirmatory clinical trial.
For Revive’s macro pathway-to-commercialization, there is a knock-on effect.
Updating its IND with the FDA for Bucillamine, creates a foundation to “pursue future programs with Bucillamine in infectious diseases and inflammatory and respiratory disorders.”
The FDA IND package is expected to be submitted in June 2020. Revive anticipates, “obtaining FDA acceptance to proceed to a Phase 3 study”.
The Company is also preparing its pre-Clinical Trial Application package to Health Canada that will “include data on the safety, efficacy, manufacturing process and clinical trial protocol of Bucillamine”.
Revive expects to have feedback from Health Canada in June 2020, which could lead to regulatory clearance to to initiate a clinical study.
For a biotech company, these milestones are highly significant.
The stock price of a drug developer typically goes up as its drug gets green-lit through clinical trials.
The markets are so sensitive to these calibrated triumphs, that a stock will surge on a declaration that something bad didn’t happen.
Example: CymaBay Therapeutics (CBAY.NASDAQ)’s share price doubled in the last 48 hours, after announcing its drug Seladelpar “does not cause liver injury”.
 Drug Delivery License and Psilocybin Research and Development:
Drug Delivery License and Psilocybin Research and Development:
Revive has entered into a “sponsored research partnership with the University of Wisconsin-Madison to evaluate novel formulations and drug delivery technology focused on psilocybin-based pharmaceuticals”.
Revive has expanded its exclusive license of the drug delivery technology from the Wisconsin Alumni Research Foundation (WARF) to include all hallucinogenic compounds.
Revive has a worldwide license agreement with WARF to commercialize all cannabinoids and hallucinogenic compounds using the drug delivery technology.
This includes the delivery of synthetic and natural extracts of psilocybin in topical gels, creams, ointments, oral, transdermal patches, oral and foams.
Revive is currently evaluating novel oral dosage forms of psilocybin, such as oral dissolvable thin films or tablets. The company intends to “pursue clinical studies for indications currently not being evaluated with psilocybin”.
Revive is also exploring opportunities to sponsor an investigator-led clinical trial evaluating psilocybin in the treatment of a particular indication “to be disclosed once an agreement has been finalized”.
In this May 6, 2020 Proactive Investor’s interview, Frank and Pharma Founder Derrick Welsh talk about the Phase 3 clinical trial and Revive’s objectives in the psilocybin space.
“Right now, Revive is one of the early entrants to the burgeoning shrooms/psychedelics/psilocybin industry, a business that is so early it’s not yet legal,” wrote Chris Parry on March 20, 2020, “You could roll your eyes at that, but there are plenty of indicators to suggest the shroom movement will land quicker than medical marijuana did”.
Revive is sitting in a sweet spot.
In an industry where drug development can stall for years, its product pipeline is gushing.
Full Disclosure: Revive Therapeutics is an Equity Guru marketing client.