Tetra Bio-Pharma (TBP.T) announced that their new investigation new drug (IND) ARDS-003 has been studied in a viral infected organoid model as part of a research collaboration between Targeted Pharmaceuticals and George Mason University.
ARDS-003 is a therapeutic developed to treat hyperinflammatory conditions, which has been a big part of COVID-19 sickness. ARDS-003 is patent protected. The research collaboration between Tetra and Targeted was announced back in March.
ARDS is among the most common conditions leading to death for those with COVID-19, after pneumonia.
ARDS stands for Acute Respiratory Distress Syndrome, which is a type of respiratory failure characterized by rapid onset of widespread inflammation in the lungs. ARDS-003 is also designed to treat sepsis, which is a life-threatening reaction to an infection that happens when your immune system overreacts to an infection and starts to damage your body’s own tissues and organs.
As this paper in Nature shows, ARDS is among the most common conditions leading to death for those with COVID-19, after pneumonia. Septic shock is among the top causes of death for those who died from COVID-19.
“Our partnership with Targeted is allowing us to learn more about the therapeutic potential of our proprietary drug ARDS-003. Sepsis is a major cause of death worldwide and viral infections contribute to this. These types of studies are a first of a kind using neuro-organoid spheres. We believe this partnership will lead to new market opportunities for both corporations as we leverage our cannabinoid inhalation intellectual property as well as that of the ARDS-003 drug,” stated Dr. Guy Chamberland, CEO and Chief Regulatory Officer of Tetra Bio-Pharma.
“[ARDS-003] clears the virus significantly better than the standard antiviral drug alone.”
Today’s press release says the research by Targeted shows “[ARDS-003] clears the virus significantly better than the standard antiviral drug alone.” Extracellular vesicles (EVs) play a role in the immune response against viral pathogens and post-infection complications by incorporating and spreading both viral and host factors, thereby inducing or inhibiting immune responses towards the viruses via multiple mechanisms, and studies have shown that EVs are implicated in the SARS-CoV-2.
“These studies are significant in that cannabinoids may provide a protective effect by alleviating the pathogenic effects of EVs in HIV-1 and CNS-related infections,” commented Dr. Fatah Kashanchi, Professor and Director at the George Mason University’s Laboratory of Molecular Virology Lab. “Our data suggests that certain cannabinoids, such as CBD and THC can act as viral transcription inhibitors, potentially through two independent mechanisms and provide significant reduction in EVs released from infected cells”, stated Dr. Kashanchi in a June 16 press release. Tetra confirms that this research also included its proprietary investigational drug ARDS-003.
“Understanding the affect of ARDS-003 on EVs in viral infections is important for guiding the long-term clinical development of Tetra’s proprietary asset, particularly since this drug clearly has both anti-inflammatory and antiviral properties. The neuro-organoid spheres used in this study are like mini brains! They get infected with the virus thereby allowing us to study our drug without infecting live animals. This has transformed how we conduct research” stated Dr. Chamberland.
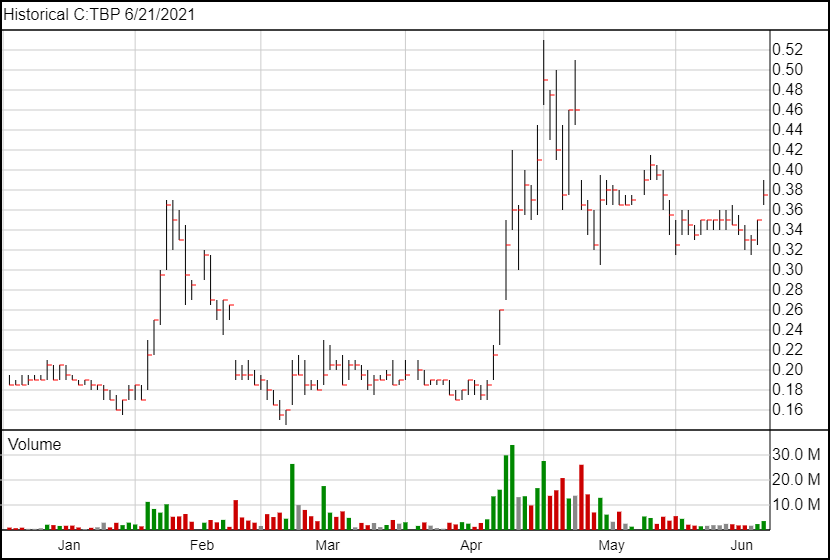
Following today’s news, Tetra Bio-Pharma’s shares are up 3 cents and are currently trading at $0.38.