AzurRx BioPharma (AZRX.Q) today announced positive topline results from its Phase 2 Combination Trial evaluating MS1819 in combination with the current standard of care, porcine-derived pancreatic enzyme replacement therapy (PERT), for the treatment of severe exocrine pancreatic insufficiency in patients with cystic fibrosis.
“This is a positive day for AzurRx, and with data from all 20 study participants in hand, our enthusiasm for the MS1819 program remains undiminished…Topline data clearly show that combining MS1819 to the daily dose of PERT had clinical benefits for all patients and improved quality of life,” stated James Sapirstein, President and CEO of AzurRx.
There’s a lot to breakdown here, but let’s start with AzurRx. AzurRx is a clinical stage biopharmaceutical company specializing in the development of targeted, non-systemic therapies for gastrointestinal (GI) diseases. The Company’s lead product candidate is MS1819, a recombinant lipase for the treatment of exocrine pancreatic insufficiency (EPI) in patients with cystic fibrosis and chronic pancreatitis. For context, which we are going to need a lot of, lipase refers to an enzyme primarily produced by the pancreas to help digest dietary fats. With this in mind, EPI is a condition characterized by a deficiency of the exocrine pancreatic enzymes, including lipase. So, where does cystic fibrosis fit into this equation? Cystic fibrosis (CF) is an inherited genetic condition that leads to chronic diseases that mainly affect the lungs, digestive, and reproductive systems. As you probably could have guessed, EPI is a major complication of CF. In fact, CF is the second most common cause of EPI, after chronic pancreatitis.
Before we move on, let’s talk about PERT, the current standard of care for EPI treatment in patients with CF. PERT involves the consumption of enzyme supplements containing a mixture of pancreatic enzymes, lipase, amylase and protease that assist the digestion of fat, carbohydrates and proteins. However, unlike PERT which relies of enzymes extracted from pig pancreas glands, MS1819 utilizes Yarrowia lipolytica yeast lipase to break up fat molecules in the digestive tract of EPI patients. For the sake of time, I won’t open that can of worms. All you need to know is that the Company’s Yarrowia lipolytica yeast lipase, in combination with PERT, was able to achieve primary and secondary endpoints in a Phase 2 Combination Trial. In particular, data collected from 20 patients indicated that MS1819 in combination with PERT led to clinically meaningful improvements in fat absorption. Additionally, the study also demonstrated positive improvements in weight gain and other secondary endpoints.
According to the AzurRx’s Q2 Financial Results, the Company’s cash and cash equivalents increased from $6,062,141 on December 31, 2020 to $8,074,491 on June 30, 2021. With that being said, the Company’s total assets also increased from $12,923,855 to $13,776,799 in the same period. On the contrary, AzurRx’s total liabilities were reduced significantly from $15,564,548 to $6,739,875. However, the Company reported a net loss of $9,278,944, which was largely driven by increased operating expenses. In total, AzurRx’s loss from operations increased substantially from $2,393,704 on June 30, 2020 to $9,276,888 on June 30, 2021. With this in mind, the Company has incurred recurring losses, experience recurring negative operating cash flows, and requires significant cash resources to execute its business plans. However, AzurRx has an extensive pipeline of products currently undergoing various clinical trials. If successful, these products could provide the Company with the substantial returns it needs.
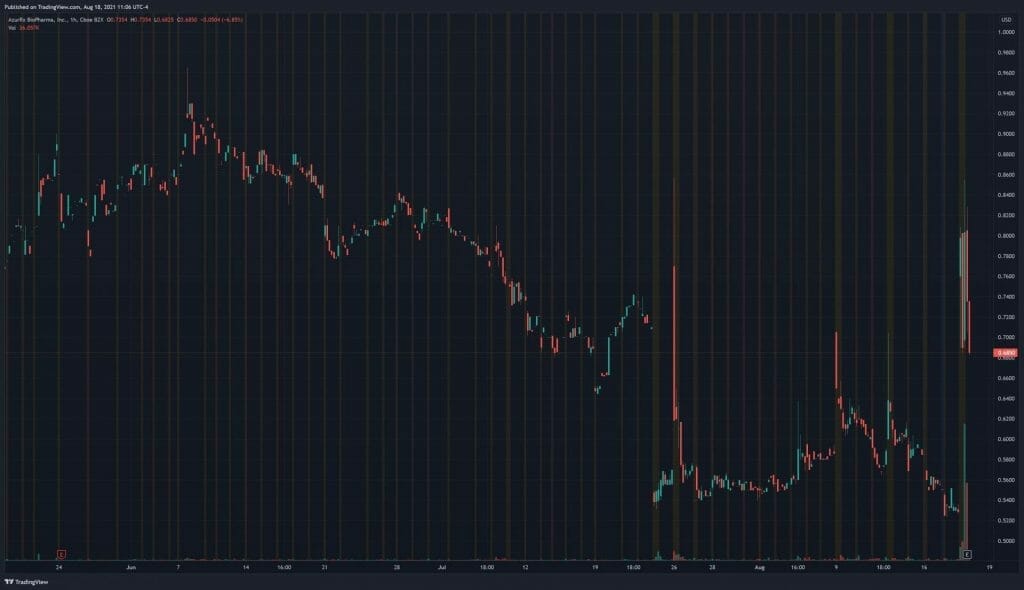
AzurRx’s share price opened at $0.71, up from a previous close of $0.527. The Company’s shares are up 31% and are currently trading at $0.698 as of 11:07AM ET.

