Ardelyx (ARDX.Q) announced today that it has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) regarding the Company’s New Drug Application (NDA) for tenapanor.
“We are saddened by this communication from the FDA and what it means for the patients and the physicians who treat them…We continue to believe tenapanor represents an important, first-in-class treatment option for patients with elevated phosphorus,” said Mike Raab, president and chief executive officer of Ardelyx.
Ardelyx is a biopharmaceutical company focused on the discovery, development, and commercialization of innovative medicines to improve treatment for people with kidney and cardiorenal diseases. With this in mind, Ardelyx is credited for the development of tenapanor for the treatment of hyperphosphatemia. Hyperphosphatemia is an electrolyte disorder in which there is an elevated level of phosphate in the blood. This can result in a range of symptoms including muscle cramps, spasms, joint pain and weak bones. Furthermore, hyperphosphatemia is a nearly universal condition in more than 550,000 Americans with Chronic Kidney Disease (CKD) on dialysis, and is a major risk factor for cardiorenal morbidity and mortality.
Currently, phosphate binders are the only available class of therapy for the treatment of hyperphosphatemia, yet a significant proportion of patients are still unable to achieve and maintain target phosphorus levels. With this in mind, Ardelyx’s tenapanor is intended to function as a phosphate absorption inhibitor. To date, the Company has conducted three Phase 3 clinical trials evaluating the safety and efficacy of tenapanor for the control of serum phosphorus in adult patients with CDK on dialysis. In all three clinical trials, tenapanor met its primary and secondary endpoint, demonstrating efficacy is reducing phosphorus. Sounds great, right?
Well, according to the CRL, while the FDA acknowledges that the submitted data provides substantial evidence of tenapanor’s effectiveness, they have characterized the magnitude of its effects as small and of unclear clinical significance. Furthermore, the FDA notes that in order for Ardelyx NDA application to be accepted, the Company needs to conduct an additional trial demonstrating a clinically relevant treatment effect of serum phosphorus. On the bright side, there were no safety, clinical pharmacology/biopharmaceutics, CMC or non-clinical issues identified in the CRL. Additionally, the FDA indicated that it is willing to meet with Ardelyx to discuss options for obtaining approval. With this in mind, the Company plans to request a Type A meeting as soon as possible.
We do not agree with the FDA’s subjective assessment on the clinical relevance of the treatment effect of tenapanor in our studies which met all clinical endpoints agreed upon by the FDA. In our view, the serum phosphorus lowering data generated with tenapanor in all of our clinical studies is meaningful and clinically significant. We will work with the agency to address the issues raised and, to the extent possible, find an expeditious path forward,” continued Mike Raab.
According to Ardelyx Q1 2021 results, the Company’s total assets were $210,293,000, with total liabilities of $79,482,000 as of March 31, 2021. At the end of the same period, the Company’s cash and cash equivalents were $84,070,000, however, in Ardelyx’s latest press release, the Company has disclosed that as of June 30, 2021, it had $171,800,000 in cash and cash equivalents. Having taken quite the tumble following the FDA’s initial rejection of Ardelyx’s NDA for tenapanor, the Company has quite a ways to climb before reclaiming its previous share price of over $7.00.
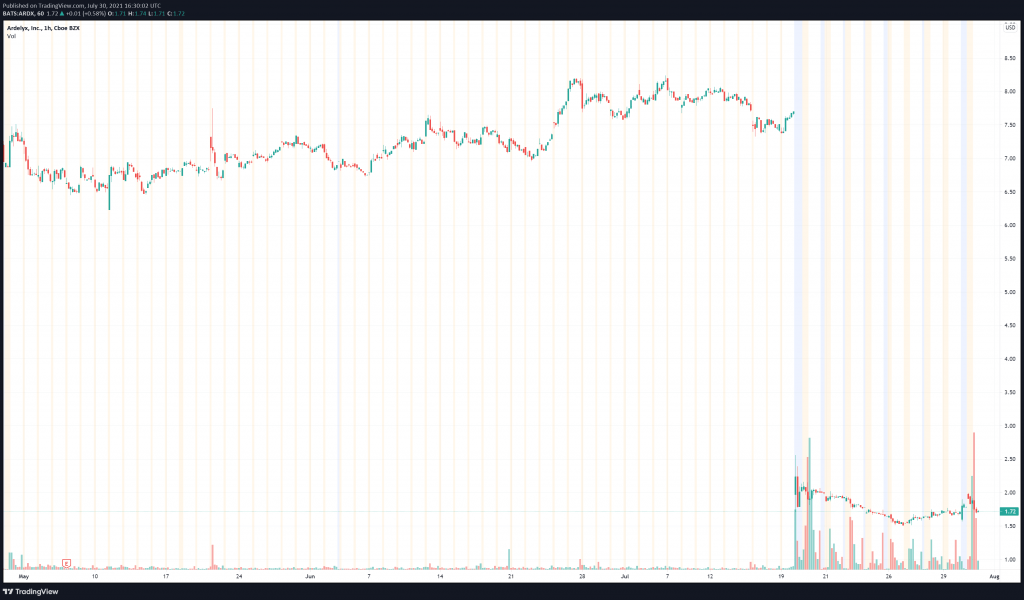
Ardelyx’s share price opened at $1.76, up from a previous close of $1.71. The Company’s shares are up 1.16% and are currently trading at $1.73 as of 12:41PM ET.


