Numinus Wellness (NUMI.V) announced that Health Canada has cleared their MAPS-sponsored single-arm, open-label safety and feasibility study evaluating MDMA-assisted therapy for post-traumatic stress disorder (PTSD).
The study is being done in collaboration with Multidisciplinary Association for Psychedelic Studies’ (MAPS) wholly-owned subsidiary MAPS Public Benefit Corporation (MAPS PBC). MAPS says that MAPS PBC serves as a vehicle for conducting MAPS’ research, and aims to balance social benefits with income from the legal prescription sale of MDMA. MAPS PBC plans to file their MDMA PTSD treatment for US FDA approval in 2023.
Study preparations are taking place at Numinus’ Vancouver clinic and are moving towards the final stages, which includes training staff, importing medication and obtaining ethical approval to allow the recruitment of participants according to the current COVID-19 public health protocols. The study is aiming to assess the safety and effectiveness of MDMA-assisted therapy.
“We are thrilled that Health Canada has issued its No Objection Letter allowing this important study to proceed and, in doing so, potentially advance Canada toward a legal, regulated system for MDMA-assisted therapy,” said Payton Nyquvest, CEO of Numinus. “At Numinus, we are focused on expanding patient access to psychedelic-assisted therapies such as MDMA for PTSD, and we are gratified that our study will provide safety and outcome data to regulators to support integration of this treatment into mainstream mental health care.”
According to research, the “prevalence rate of lifetime PTSD in Canada was estimated to be 9.2%, with a rate of current (1-month) PTSD of 2.4%.” This means that nearly one in ten Canadians will experience PTSD within their lifetimes, and just under one in forty are currently experience PTSD. Even just the productivity loss from PTSD in Canada is estimated at $21 billion each year.
MDMA has been gaining attention as a treatment for PTSD, even reaching the pages of The New York Times recently. In May, a paper was published in the prestigious journal Nature which stated that “MDMA-assisted therapy is highly efficacious in individuals with severe PTSD, and treatment is safe and well-tolerated, even in those with comorbidities” after a phase III study produced positive results.
“As research into MDMA-assisted therapy grows, it is critical that we develop data on outcomes from a diverse, real-world array of clinical environments,” stated Amy Emerson, CEO of MAPS PBC. “Clinical studies conducted for the purpose of regulatory approval often address research questions focusing on safety and efficacy of the treatment. This collaborative demonstration study with Numinus will build upon MAPS-sponsored multi-site studies which enrolled some Canadian participants and will provide new and comprehensive information about the effectiveness of MDMA-assisted therapy for populations with PTSD and concurrent disorders.”
Numinus’ Medical and Therapeutic Services Director Dr. Christie will serve as the study’s Qualified Investigator.
“Health Canada should be recognized for its ongoing leadership through its support of this study,” stated Dr. Christie “At our Vancouver clinic, we have spent months establishing the physical, technical, clinical and human resource infrastructure needed to move the study forward and ultimately foster greater access to MDMA-assisted therapy.”
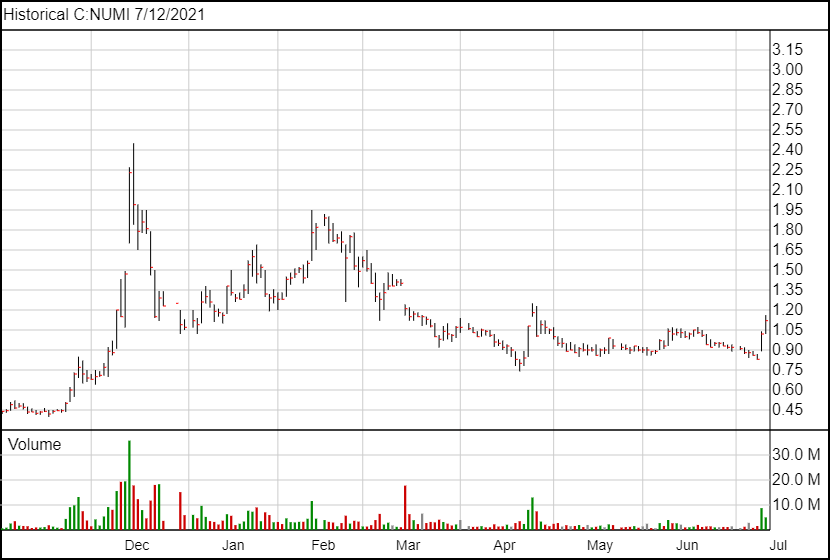
Following the news, NUMI shares are up 10 cents and are currently trading at $1.12.