Ortho Regenerative Technologies (ORTH.C) got some bad news today in the form of a clinical hold letter from the FDA related to their investigational new drug (IND) application fo ra phase 1/2 clinical trial for Ortho-R, according to a press release.
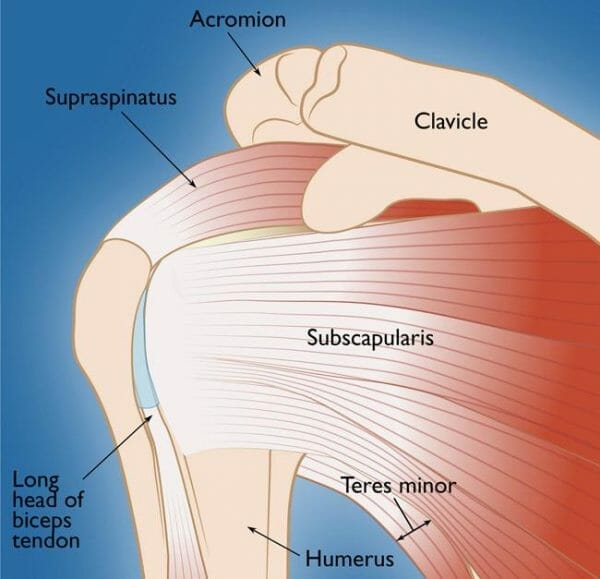
Ortho-R is a drug candidate used in conjunction with standard of care surgery in the case of rotator cuff tear repair.
“We appreciate the FDA’s guidance and assistance to Ortho, in the development of a first-in-class orthobiologics combination product to improve outcomes of standard of care surgery in rotator cuff tear repair. We will work diligently to address the FDA’s questions as quickly as possible and look forward to continuing to work closely with them to secure IND approval. In parallel, we will continue working on our phase I/II clinical trial preparation activities to ensure we minimize the impact on our overall timelines,” said Claude LeDuc, president and chief executive officer of Ortho.
Ortho is a clinical stage orthobiologics company involved in the development of novel therapeutic soft tissue repair technologies to improve success rates of orthopedic and sports medicine surgeries. The company’s Restore technology platform is a drug delivery system that facilitates the delivery of biologics like platelet-rich plasma or bone marrow aspirate concentrate to help regenerate new tissue in musculoskeletal conditions.
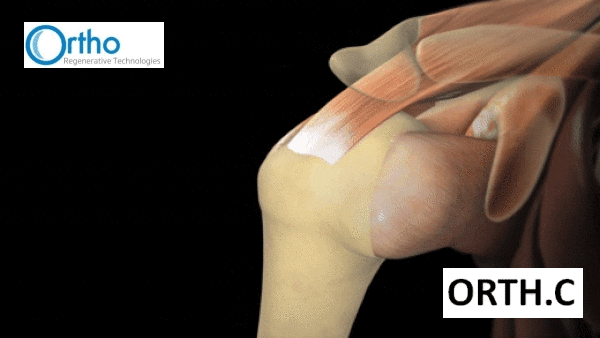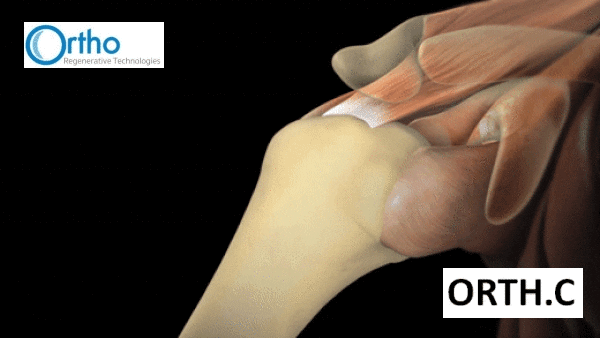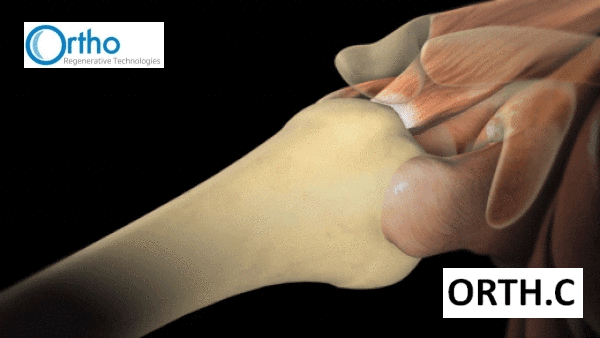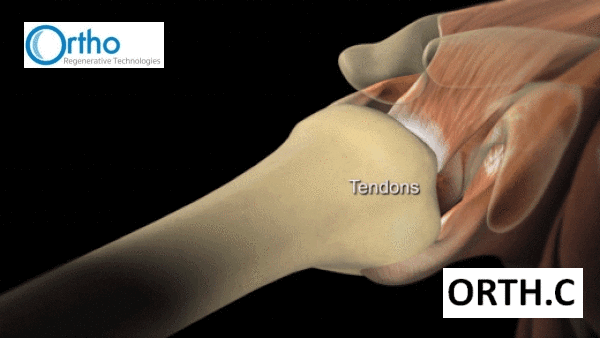
If your MD is in the mail and you’re unfamiliar with all the ligaments, muscles and tendons of the body, the rotator cuff is the name for the four tendons that stabilize the shoulder joint. It’s a common sports injury, but it can also be caused by repetitive overhead tasks, like pulling a boxes down off a shelf for eight hours, or maybe getting caught in a nasty armlock by someone who doesn’t know what they’re doing. Not a fan.
The only non-surgical way out is through conservative therapy. The principle aim of said surgery is to reattach the torn tendon to the bone. Standard of care involves using suture anchors which are placed into the bone while the tendon is held in place with sutures. Four million Americans get these nasty injuries and all are at risk of being disabled. It’s estimated also that a quarter of American adults over 40 will get a rotator cuff tear, exacerbated by age and lifestyle.
As for Ortho, the FDA sent back a request for additional chemistry, manufacturing and control related data and characterization, which is something the company is confident they’ll be able to provide in the coming few weeks, so they have continued preparing for the clinical trials in preparation for IND approval.
—Joseph Morton
Full disclosure: Ortho Regenerative Technologies is an equity.guru marketing client.