XPhyto Therapeutics (XPHY.C) announced they are beginning a pilot project with their rapid Covid-19 PCR test (Covid-ID Lab) in a point-of-care (POC) setting in Germany.
The Covid-ID Lab test was approved by the European Union in March, and provides PCR level Covid-19 diagnostic results within 25 minutes. The pilot project will further optimize the test’s workflow. XPhyto is partnering with Spitzweg Apotheke, a pharmacy in Langen, near Frankfurt, who are currently running a COVID-19 test center at a clinic.
“With a specialized oncology pharmacy, many of our customers are in the COVID-19 high-risk group. For these patients, their families and their close contacts, fast and reliable diagnostics are critical to ensure everyday safety,” commented Gabor Perl, Head of the Spitzweg pharmacy. “PCR tests are the diagnostic gold standard for COVID-19. They provide high sensitivity and specificity. We are pleased to take part in this pilot project and now offer our high-risk-group customers access to a rapid PCR test with immediate results. We believe this is an opportunity for best-in-class healthcare delivery.”
In their press release, XPhyto said that all the necessary lab equipment for the pilot project is installed and operational and that all those involved in the project have completed the necessary training.
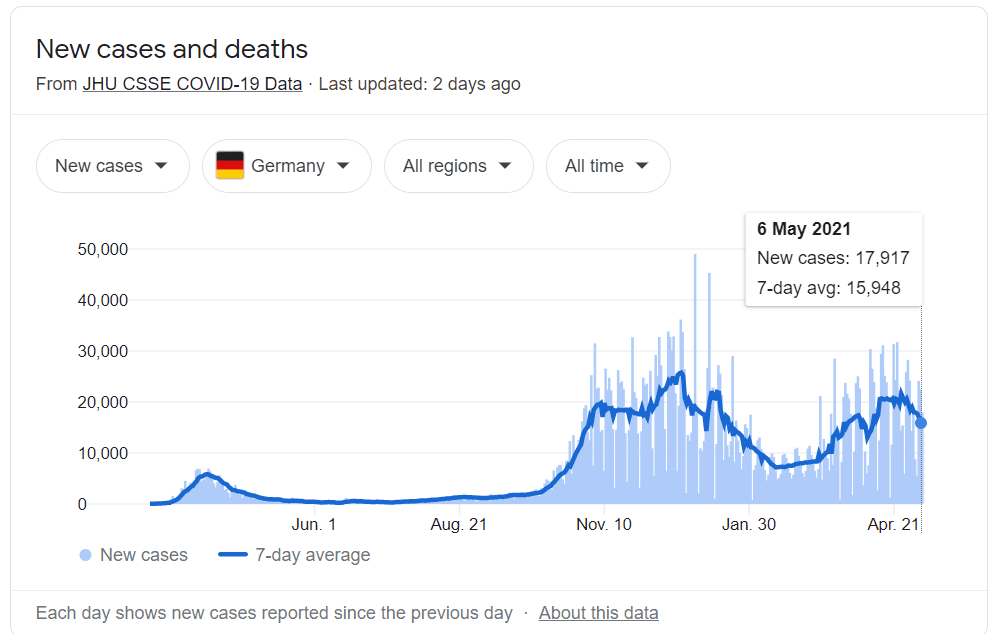
XPhyto’s Covid-ID Lab tests provide a valuable resource to Germany in the fight against Covid-19, who reported over 17,000 new cases of Covid-19 yesterday. On Wednesday, they announced they had https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered at least one dose of the vaccine to 30% of their population.
Because of the Covid-ID Lab’s high accuracy and rapid test time, it could have important uses even as cases decline, helping Germany open up their economy. On top of its speed and efficacy, the Covid-ID Lab is not that difficult to use. Many widely available standard PCR instruments are suitable to run the test, meaning testing clinics will not need to purchase new equipment to use the tests. Additionally, the results are collected and shown on an easy-to-read platform.
By optimizing the test’s workflow and adapting it to a POC setting, XPhyto is increasing the situations in which the test can be effectively deployed.
The Covid-ID Lab test was also able to detect the variant strains of Covid-19.
“Offering Covid-ID Lab to patients in Germany is a significant milestone. The test has gone from concept to commercial use in less than twelve months,” said Hugh Rogers, XPhyto CEO and Director. “Covid-ID Lab is designed to be one of the fastest and most portable PCR systems in the world. Adapting Covid-ID Lab to a POC setting is a major commercial opportunity for our German subsidiary XP Diagnostics.”
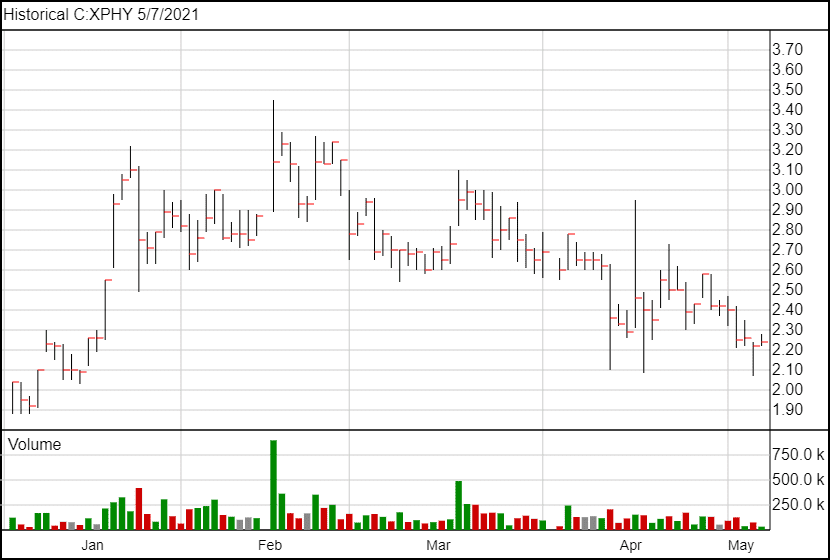
Following the news, XPhyto’s share price is up 4 cents and is currently trading at $2.24.
Full disclosure: XPhyto Therapeutics is an Equity Guru marketing client