XPhyto Therapeutics (XPHY.C) and its exclusive German diagnostics development partner, 3a-diagnostics GmbH (3a), announced they have received approval for their point-of-care Covid-19 rapid RT-PCR test system (Covid-ID Lab) for use in Europe.
After receiving their ISO certification last week, XPhyto and 3a’s Covid-ID Lab is now registered within the European Union as a commercial in vitro diagnostic (CE-IVD) test. This will allow their test to be sent out and used, and XPhyto plans on sending out tests to distributors and wholesale sellers next month. They already sent out thousands of tests to potential distributors last month.
The Covid-ID Lab will allow people to receive rapid test results, getting their results back after as quick as 25 minutes, and has a 95% confidence interval.
“Our test is one of the fastest PCR-based COVID-19 tests currently approved. With a sample collection to result time of 25 minutes, Covid-ID Lab combines the speed of a rapid screening test with the accuracy of a PCR diagnostic,” said Hugh Rogers, CEO and Director of XPhyto. “Covid-ID Lab is designed for point-of-care testing, particularly in satellite and small-scale labs, such as transportation hubs, borders, care facilities, schools, pharmacies, and hospitality settings.”
Because of its speed and high confidence interval, the Covid-ID Lab provides users with many advantages. For diagnostic performance, Covid-ID Lab requires only a single 20-minute PCR thermal cycle without prior RNA extraction as part of the sample preparation. RNA extraction can create major testing bottlenecks, and so XPhyto’s RT-PCR test – which doesn’t require RNA extraction – can help deal with this bottleneck.
Additionally, many widely available standard PCR instruments are suitable to run the test. This means those wanting to https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngister these new tests won’t need to purchase new equipment to run these tests.
Results are collected and shown on easy-to-read optical indicator strips on a simple fluidics platform.
The combination of ease of https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration, minimal equipment needed, rapid results, and easy-to-read results make this test suitable for a variety of situations. The Covid-ID Lab test could be used in a variety of circumstances as countries attempt to loosen restrictions while keeping the Covid-19 pandemic under control.
However, the statement made clear that “The Company is not making any express or implied claims that its product has the ability to eliminate, cure or contain the COVID-19 pandemic.”
The test also detected, with 100% specificity, 19 other pathogens of serious respiratory infections on a respiratory panel. This news shows that the technology can be used going forward once we can (finally) begin to move past the Covid-19 pandemic.
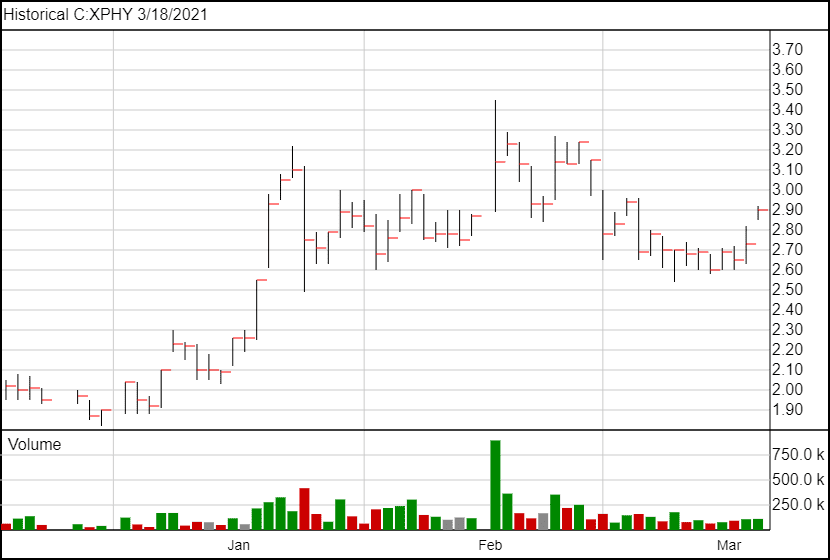
Following the news, XPHY opened up 16 cents higher than the previous days close, and is currently trading at $2.90.
Full disclosure, XPhyto Therapeutics is an Equity.Guru marketing client.