Earlier this month I wrote a piece on Field Trip (FTRP.C) scaling their ketamine clinics after a huge $95M raise. I liked their business model, and Novamind’s (NM.C) is similar, but with a much smaller market cap.
Novamind has two main divisions, clinics and research. The company provides ketamine-assisted psychotherapy and other novel treatments through its network of Cedar Psychiatry clinics which serve in 4 locations in Utah, with a 5th coming soon.
60 years of ketamine
Ketamine emerged in the medical community in 1962 through Parke-Davis Labs, a company that was acquired by Pfizer (PFE.N). The FDA approved ketamine for human use in 1970 and was a popular anesthetic given to American soldiers during the Vietnam War. After an increase in recreational use in 1999 the DEA listed ketamine as a Schedule III drug under the CSA. However, under U.S. federal law, ketamine can be prescribed to a patient at a doctor’s discretion.
Novamind’s Cedar Psychiatry physicians prescribe and https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngister Spravato, a derivative formulation of ketamine that was approved by the FDA in 2019. They are also licensed to prescribe medical cannabis, the primary indication being PTSD.
https://equity.guru/2021/03/11/why-companies-like-mindset-pharma-mset-ne-are-adamant-on-synthesizing-psilocybin-mushrooms/
In its other division, the company operates Cedar Clinical Research – a Utah-based contract research organization specialized in clinical trials and evidence-based research for psychedelic medicine. They specialize in hosting phase I to phase IV clinical trials and research focused on treatment options in neuropsychiatry. They are currently doing clinical studies on:
- Tourette syndrome
- borderline personality disorder
- PTSD
- negative symptoms of schizophrenia
They have done extensive tests on ketamine in the past for major depressive disorder, eating disorders, end of life therapy, and psychotherapy. They also conduct clinical trials as a third party as part of their previous stated revenue model.
Novamind is expanding both divisions of the company with $5M CAD allocated towards scaling their mental health clinics, and for participation in psychiatric clinical trials. Novamid anticipates increasing its number of clinics and clinical research sites both through organic growth and through acquisitions. Estimated capital expenditure for a new clinic is in the range of $100,000 to $150,000.
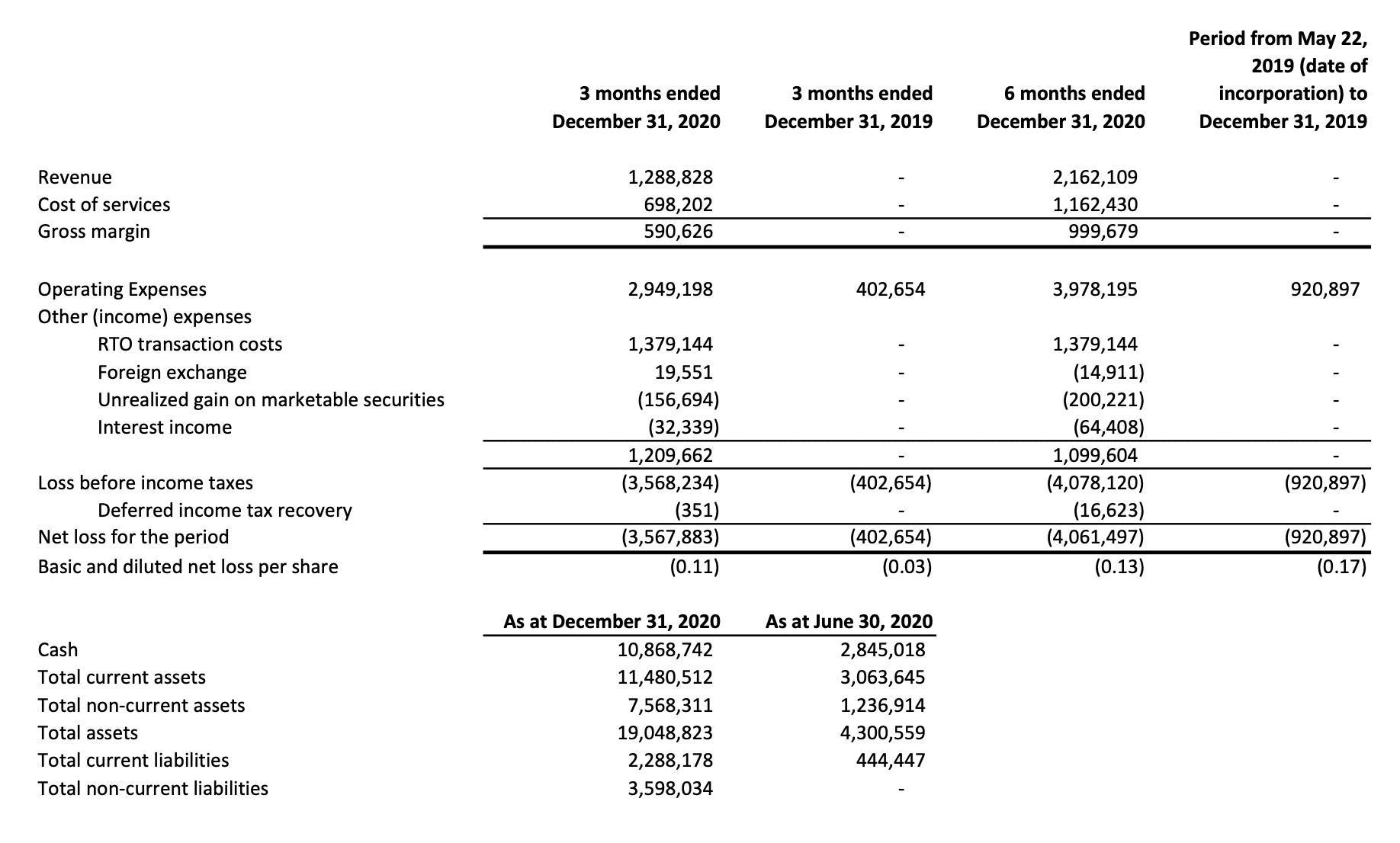
The company completed a $10M CAD raise last November and has raised a total of $15M CAD to date. As of December, the company had $9.2M CAD in cash.
The company’s budget (shown above) for the next 12 months is exactly what their current cash position is, so they’re going to need to do more raises – or rely on significant revenue growth in the short term. Psychedelics is a cash-intensive business and it looks like management is prepared for that. They have cannabis OG Chuck Rifici on their board from Auxly Cannabis (XLY.C), the company’s stock might have gone sideways but the dude knows how to raise a buck. He was also one of the founding members of the cannabis giant Canopy Growth (CGC.N), he got in early to cannabis and is now doing the same with psychedelics.
Revenue
Last year Novamind generated $2.1M CAD in revenue through outpatient mental health services, (namely ketamine-assisted psychotherapy) with a gross margin of $1M CAD. The company also earned income through hosting clinical trials for emerging treatment options in neuropsychiatric on behalf of third-party sponsors, generating $53,943 CAD in revenue last year.
That’s impressive for a newer company in the space just getting started, and 2020 was a down year for most businesses, namely brick and motor. When the pandemic caused shutdowns around the globe, retail foot traffic dropped as The U.S. had an 82.6% year-over-year foot traffic plunge.
Over half of businesses in Canada reported a revenue decrease compared to August 2019, irrespective of their employment size. It was normal for smaller businesses to report a revenue decrease of 40% or more.
Novamind is a relatively small company, but the psychedelics sector is not shrinking, and with depression and anxiety rates through the roof because of COVID, a company with a clinic network could increase its revenue and margins significantly. People are going to come out of this thing needing treatment more than ever.
MDMA next?
I don’t think we can truly calculate predictable future revenue based on ketamine alone. Once more drug choices are available it will bring in a larger range of clientele, and potentially create even more loyal repeat customers who want to try new drugs and have new experiences. Someone willing to do psilocybin therapy might be opposed to ketamine therapy.
There is a natural difference in opinion among consumers, but also decades of anti-drug propaganda that has hidden the benefits of certain drugs while exaggerating the negatives. These cognitive biases exist for people seeking therapy, unfortunately. MDMA, a much more widely known drug is showing huge promise, and Novamind is looking forward to when they can use it in their clinics.
A major milestone for MDMA therapy was The Multidisciplinary Association for Psychedelic Studies (MAPS) being granted Breakthrough Therapy Designation in August 2017 for its MDMA-assisted psychotherapy clinical trial studying the treatment of PTSD.
In MAPS’ phase II clinical trials it was found that the benefits of MDMA-assisted psychotherapy for PTSD extended at least 12 months after the treatment. These trials have shown that MDMA can reduce fear and defensiveness, enhance communication and introspection, and increase empathy and compassion, enhancing the therapeutic process for people suffering from PTSD.
In a long-term follow-up study, it was found that 67% of participants no longer qualified for a PTSD diagnosis. MAPS’ phase III clinical trials are expected to be completed in 2022, with FDA approval possible as early as 2023.
Novamind has a good connection with MAPS. Director and CMO of Novamind Dr. Reid Robison is currently the coordinating investigator for the MAPS-sponsored MDMA-assisted psychotherapy study of eating disorders.
It’s hard to really value this company yet as is true with any psychedelics company at this point. That being said, I like the clinic business model, and a new company without an established client base putting up those numbers during COVID is nothing to scoff at.
At this point, they are only allowed to prescribe ketamine and cannabis, but that will open up as rules and regulations change and when that happens I would expect their numbers to go up.