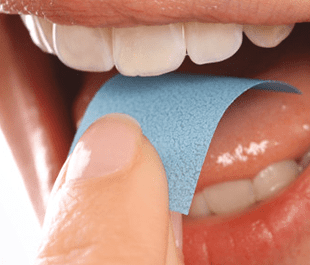
Xphyto Therapeutics’ (XPHY.C) subsidiary Vektor Pharma TF GmbH has finished work on their oral disintegrating film (ODF), a dosage formulation for a major European pharma-company and is now ready to push it through clinical trials, according to a press release.
The formulation is designed to quickly adjust to various active pharmaceutical ingredients (APIs). The company can vary the product formulations in their lab in order to meet dosage requirements set by the client company. It’s based on a target drug and the company’s specifications for dosage profile to control the mechanical properties of the ODF, such as disintegration rate and drug release properties.
“Our ODF drug delivery platform provides a fast-track opportunity to bring new drug formulations to market. Xphyto is developing a pipeline of new dosage formulations based on approved APIs but Vektor is also working for drug companies as a development and manufacturing partner. Vektor can provide its clients with a versatile and rapidly adjustable ODF and transdermal dosage platform to create new delivery options for generic and non-generic APIs,” said Dr. Thomas Beckert, Vektor’s managing director.
Vektor has worked for both major and minor drug companies over the past ten years in developing new dosage formulations using their ODF and transdermal drug delivery systems. Applicable drugs the technology will and has worked on include fentanyl, rivastigmine, clonidine and rotigotine, but also including non-generic active formulations for maladies such as Parkinson’s disease, restless leg syndrome, incontinence, local pain and abuse-resistance pain medications.
According to Market Data Forecast, the global thin film segment of the drug market is anticipated to expand to USD$29.2 billion by 2024 at a compound annual growth rate of 10.5%.
—Joseph Morton
Full disclosure: Xphyto Therapeutics is an equity guru marketing client.