BrainStorm Cell Therapeutics (BCLI.Q), a biotech company involved in a research study for amyotrophic lateral sclerosis (ALS), received a combined $500,000 grant from The ALS Association and I AM ALS today.
The purpose behind the grant was to support their ongoing phase 3 clinical trial of their NurOwn treatment, which aims to widen the understanding of critical biomarkers with treatment response for people with the disease.
“This grant to BrainStorm marks an important step forward in establishing how exactly NurOwn works in the body. This research is also important to our overall pursuit of identification and validation of ALS biomarkers. We hope NurOwn is ultimately proven effective in treating ALS and we stand ready to support BrainStorm in its plan to apply for a biologics license for NurOwn,” said Calaneet Balas, president and CEO of The ALS Association.
ALS is a progressive nervous system disease that affects nerve cells in the brain and spinal cord, causing a loss of muscle control. It’s often called Lou Gehrig’s disease after the legendary baseball player from the 1930’s who was diagnosed with it. Doctors don’t yet know why ALS occurs, but they have determined that there is a genetic factor, as some cases are inherited. The disease begins with muscle twitching and weakness in a limb or slurred speech. Eventually, the disease causes the loss of the ability to move, speak, eat and breathe normally. As of this time, there is no cure.
To be more specific about the study, it designed to determine how NurOwn interacts with its targets in the brain and spinal cord and to explore changes in the biomarkers that may correlate with response to the drug treatment. Two examples of biomarkers are cholesterol or blood pressure, but it’s really any measureable substance in the body that can change over time. If the study is successful, it will assist with confirming that the NurOwn works the way it’s supposed too, and will help the company develop their understanding of ALS biomarkers.
The grant is split up into two, with The ALS Association offering the lion’s share at $400,000 and I AM ALS giving $100,000. The agreement comes with one stipulation—that BrainStorm agree to share data and samples with the LS community so that results can be independently validated, as well as to advance other ALS research.
“We need to move with urgency in all of our efforts to deliver treatments and cures for ALS. This biomarker research will help us more expeditiously understand the effectiveness of NurOwn, while possibly unlocking discoveries that provide clues for other promising treatments. We are at a pivotal time for ALS research in pursuit of treatment solutions and will do whatever we can, together, to drive new answers and new hope for patients,” said Danielle Carnival, CEO of I AM ALS
It’s a good time to be into biotech. With plenty of other industries either shut down or in the process of re-opening, industries designated essential services have enjoyed a critical head start. Brainstorm is among them, effecting doubling in price since the middle of March.
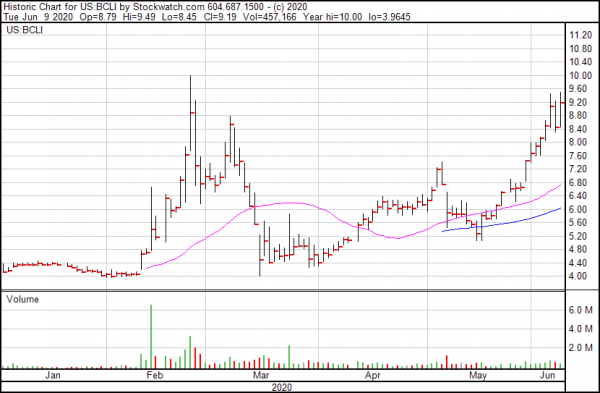
Biotechnology companies, especially those involved in clinical studies, tend to get bounces only when news comes out and then go into a long period of dormancy. Brainstorm came alive just as COVID-19 began to make the rounds, offering volatile spikes that if ridden correctly, especially on news like this, could theoretically make an investor some short term gains. Beyond that may be too much risk.
—Joseph Morton

