Positive Topline Data

- $31.886M Market Capitalization
Windtree Therapeutics Inc. (WINT.Q) announced today positive primary results with istaroxime in rapidly raising systolic blood pressure, the critical clinical objective in treating patients in cardiogenic shock. Yes, the headline was indeed a pun, however, Windtree’s shares were up 20% following open, which may be enough to put some investors into shock.
“We are extremely pleased with the results of this trial and look forward to a full presentation of the data in the coming weeks. We’re excited to be progressing the development of istaroxime in this condition,” Dr. Steve Simonson, Senior Vice President and Chief Medical Officer at Windtree.
Speaking of which, cardiogenic shock is a serious condition that occurs when the heart is failing significantly and cannot pump enough blood and oxygen to the brain, kidney, and other vital organs. According to a study published in Springer Link, 383,983 cases of cardiogenic shock were reported from 2007 to 2017. The incidence of cardiogenic shock rose by 65.6% to 44,425 cases in 2017, with a survival rate of 41.2%.
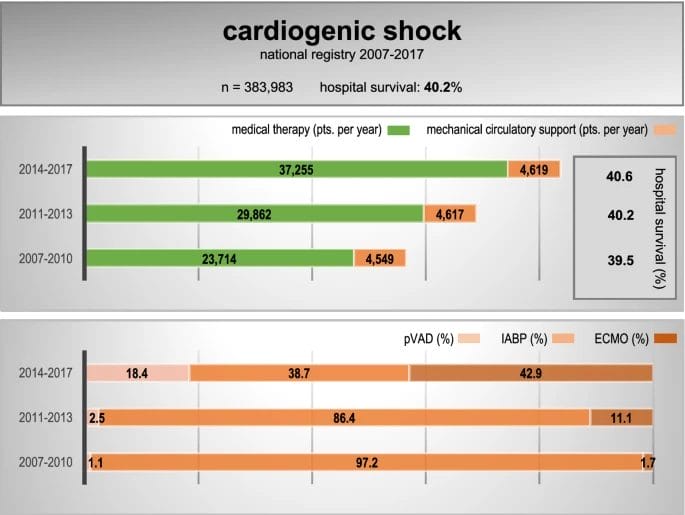
With this in mind, mortality rates associated with cardiogenic shock are significant, ranging from 7% to 40% in the United States (US). Yet, there is a lack of satisfactory pharmacological intervention to reverse the condition as available therapies have unwanted side effects, including risk for arrhythmias, decreasing blood pressure, renal dysfunction, and increases in mortality.
Needless to say, there is an apparent need for new drug innovations for the treatment of cardiogenic shock patients. In fact, market research conducted by Windtree revealed that 99% of cardiologists surveyed responded that there was a high need for new treatment in SCAI class B cardiogenic shock patients. For context, SCAI class B refers to “beginning shock” or compensated shock.
SEISMiC Study
Windtree’s SEISMiC is a Phase 2 study in early cardiogenic shock enrolling 60 patients with the SCAI class B cardiogenic shock resulting from heart failure with systolic blood pressures (SBP) between 75-90 mmHg. The study’s primary endpoint was the difference in SBP area under the curve over six hours after initiating the infusion of either Windtree’s istaroxime or placebo.
“It is worth noting the area of cardiogenic shock is complementary to our AHF program. We look forward to the next steps in our cardiogenic shock development program and meeting with regulatory agencies to further define a potential development path to approval,” said Craig Fraser, President and CEO of Windtree.
The Company’s istaroxime is a first-in-class dual mechanism therapy designed to improve both systolic and diastolic cardiac function. While I could try and explain these complicated mechanisms, I will let the results speak for themselves. To date, multiple Phase 2 studies in patients with acute heart failure (AHF) have demonstrated that istaroxime significantly improves cardiac function and blood pressure without causing heart rate increases or rhythm disturbances.
As for Windtree’s most recent SEISMiC Phase 2 study, the study met its primary endpoint in SBP over six hours, with the istaroxime treated group performing significantly better. If you would like to know more about the Company’s SEISMiC study, further details are planned to be presented at the European Society of Cardiology Heart Failure meeting to be held from May 21-24, 2022.
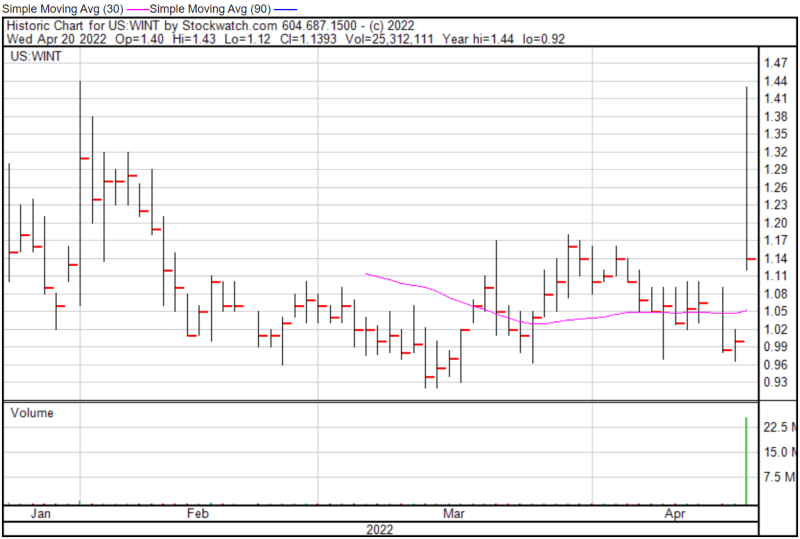
Windtree’s share price opened at $1.40 today, up from a previous close of $0.99. The Company’s shares were up 13.5% and were trading at $1.135 as of 10:50 AM ET.

