Modern Day Space Race

- $23.139M Market Capitalization
Optimi Health Corp. (OPTI.C) announced today that it has commenced the cultivation of natural GMP-grade psilocybin mushrooms at its Princeton, British Columbia (BC) facility. The Company’s initial batch is intended specifically for analysis and usage in human clinical trials, where market demand for natural psilocybin is increasing.
“Since the completion of our 20,000 square foot cultivation and processing facilities and the approval of our Controlled Drugs and Substances Dealers License by Health Canada, Optimi has pressed forward with the goal of generating revenue and demonstrating a system of safe supply that can scale with the needs of the sector while participating in research crucial to the advancement of psychedelic science,” said Optimi Health CEO Bill Ciprick.
While I can’t speak for natural psilocybin, the demand for psilocybin as a whole has increased significantly. According to Data Bridge Market Research, the Psychedelic Drug Market is projected to reach $6.85 billion by 2027, expanding at a Compound Annual Growth Rate (CAGR) of 16.3%. So, where does psilocybin fit into this equation?
In 2019, the U.S. Food and Drug Administration (FDA) granted Breakthrough Therapy designation to two psilocybin-based drugs to treat Major Depressive Disorder (MDD). To provide some context, Breakthrough Therapy is a process designed to expedite the development and review of drugs that are intended to treat a serious condition. In order to qualify, preliminary clinical evidence must indicate that a drug demonstrates substantial improvements over available therapy.
That being said, a drug that receives Breakthrough Therapy designation is eligible for all Fast Track designation features, among other benefits. With this in mind, you can bet your bottom dollar that there are 101 different psychedelic companies vying for Breakthrough Therapy designations in relation to MDD. However, to date, the FDA has only granted Breakthrough Therapy to Usona Institute and COMPASS Pathways.
Optimi’s Optimized Business

Within the psychedelics market, Optimi has gone where the money is. By utilizing a vertically integrated approach, the Company intends to cultivate, extract, process, and distribute high-quality functional and psychedelic mushroom products at its two facilities. Keep in mind, on February 7, 2022, the Company’s wholly-owned subsidiary Optimi Labs Inc. was granted a Dealer’s License by Health Canada.
The Dealer’s License allows for the possession, production, assembly, sale, and delivery of psilocybin within the regulated framework set by Health Canada. Combined with its two facilities comprising a total of 20,000 square feet, Optimi is well-positioned to meet the growing demand for psilocybin. However, this hasn’t stopped Optimi from dipping its toes into the clinical side of things.
In addition to its Health Canada Dealer’s License, Optimi has received a research exemption under Health Canada Food and Drug Regulations (FDR) for the use of psilocybin and psilocin for scientific purposes. With this in mind, Optimi is currently involved in a partnership with the IMPACT Clinical Trial Accelerator Program and has opted to assess its product prior to the submission of the Company’s clinical trial application.
“The Optimi team is 100 percent committed to helping the world deal with the concurrent crises of mental health and aging with safe, sustainable supplies of an organic product that has been used medicinally since time immemorial,” said Optimi Executive Chairman, JJ Wilson.
Furthermore, Optimi has received a Section 56 Exemption to the Controlled Drugs and Substances Act (CDSA) from Health Canada. Generally speaking, subsection 56(1) of the CDSA enables an approved medical professional to prescribe controlled substances to better treat people with treatment-resistant conditions.
Having received a Section 56 Exemption, Optimi is now able to carry out its own research at Bloom Psychedelic Therapy & Research, with a focus on determining a safe and effective standardized microdose of psilocybin for mental health conditions such as anxiety and depression, which would provide the Company with an entry point into the lucrative MDD Treatment Market.
Natural Versus Synthetic
I can think of a few scenarios where natural is infinitely better than synthetic. It’s like comparing apples to oranges or Jessica Alba to Kim Kardashian. In Optimi’s case, the Company is focused on the cultivation of natural GMP-grade psilocybin mushrooms. Compared to synthetic psilocybin, there are some cases where natural psilocybin is better for you.
For example, according to the Global Drug Survey, “magic mushrooms” are the safest recreational drug in existence. To put things into perspective, of the more than 12,000 people who reported taking psilocybin hallucinogenic mushrooms in 2016, a measly 0.2% said they needed emergency medical treatment. That’s at least five times lower than that of MDMA, LSD, and cocaine.
While natural psilocybin has demonstrated improved safety in some cases, I wouldn’t go so far as to say that natural psilocybin is superior. In terms of chemical composition, natural and synthetic psilocybin molecules are indistinguishable. However, I can understand why there would be a preference for natural psilocybin opposed to synthetic.
In relation to food, the word “natural” is a genius marketing ploy used by countless companies to promote their products. In fact, phrases like natural, organic, and fat-free, to name just a few, helped the food industry sell more than $377 billion worth of food items in the United States in 2014.
Although entirely different from the food market, I would argue the same sentimental preference towards “natural” is the same in the psychedelics market. With this in mind, companies like Optimi that are cultivating their own natural psilocybin could have a leg up in the psychedelics market. Time will tell.
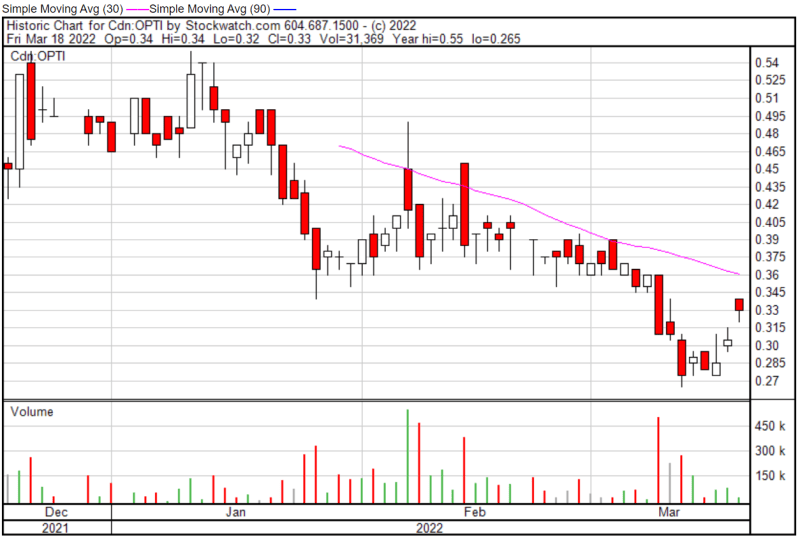
Optimi’s share price opened at $0.34 today, up from a previous close of $0.305. The Company’s shares were up 8.20% and were trading at $0.33 as of 12:17 PM EST.

