Spectrum Pharmaceuticals (SPPI.Q) announced today that it has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) regarding the Company’s Biologics License Application (BLA) for ROLONTIS®.
“We are disappointed with this outcome and look forward to fully understanding the remediation timelines for the program…We continue to believe in ROLONTIS and plan to diligently complete the regulatory process to bring ROLONTIS to market,” said Joe Turgeon, President and CEO of Spectrum Pharmaceuticals.
Spectrum Pharmaceuticals is a biopharmaceutical company focused on acquiring, developing, and commercializing novel and targeted oncology therapies. ROLONTIS®, also recognized as eflapegrastim, refers to the Company’s investigational long-acting granulocyte colony-stimulating factor (G-CSF) for the treatment of chemotherapy-induced neutropenia (CIN). Don’t worry, that didn’t make sense to me either. Apparently CIN refers to a common toxicity caused by the https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration of anticancer drugs that can result in a variety of symptoms including fever, chills, abdominal pain, and shortness of breath. CIN can also lead to febrile neutropenia (FN), which is associated with increased morbidity and early mortality, increased medical costs, and disruptions in potentially curative treatments.
ROLONTIS® has completed two Phase 3 clinical trials, both of which demonstrated the safety and efficacy of Spectrum’s investigational drug. Additionally, on April 30, 2020, the Company initiated a clinical trial to evaluate the https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration of ROLONTIS® on the same day as chemotherapy in a rat model. According to the trial, the duration of neutropenia in a rat model of CIN was observed to be significantly shorter with ROLONTIS® compared to pegfilgrastim, another drug intended to prevent neutropenia, regardless of the timing of https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration. Furthermore, data from the Company’s RECOVER and ADVANCE Phase 3 trials demonstrated that ROLONTIS® was non-inferior to pegfilgrastim in reducing the duration of severe neutropenia in all four cycles of treatment. More specifically, ROLONTIS® demonstrated a reduction of severe neutropenia of 6.5% compared to pegfilgrastim in Cycle 1 of treatment.
Spectrum applied for a BLA with the FDA back in 2018. Similar to an NDA, a BLA is required before a Company can introduce, or deliver for introduction, a biological product into interstate commerce. With this in mind, the FDA provided Spectrum with a CRL citing deficiencies related to manufacturing and indicated that a reinspection will be necessary. Further details have not been provided, however, the Company is seeking further clarification from the FDA and plans to meet with the agency as soon as possible. Getting to the point, ROLONTIS® has demonstrated non-inferiority to pegfilgrastim in numerous clinical trials. With this in mind, I think its rather disappointing that Spectrum’s stock had to take a dive following today’s news, however, the investing world is a cruel and unforgiving place.
According to Spectrums Q1 2021 results, the Company’s total assets and total liabilities were $179,007,000 and $56,568,000, respectively. As of March 31, 2021, Spectrum’s cash and cash equivalents were $69,521,000 compared to $46,009,000 in the previous quarter, demonstrating positive growth. Full details can be found here.
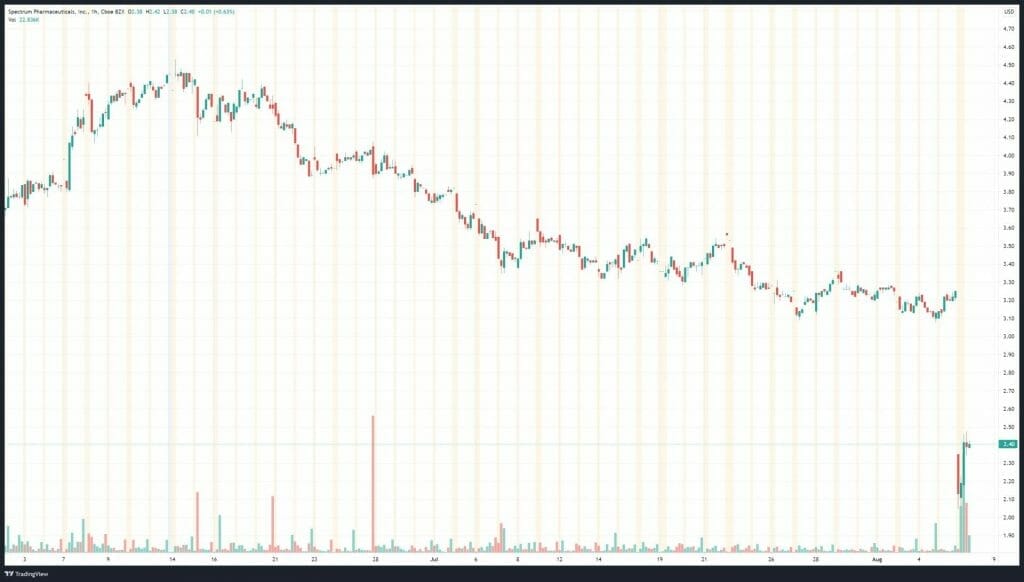
Spectrum’s share price opened at $2.29, down from a previous close of $3.25. The Company’s shares are down -26% and are currently trading at $2.40 as of 11:29AM ET.

