Iterum Therapeutics (ITRM.Q) announced today that it has received a Complete Response Letter (CRL) from the US Food and Drug Administration (FDA) for its New Drug Application (NDA). Iterum submitted an NDA for oral sulopenem, the Company’s oral antibiotic developed for the treatment of infections caused by multi-drug resistant bacteria, on July 23, 2021.
“We are disappointed in this outcome and believe that the data package submitted was adequate for the approval of oral sulopenem…Regardless, we will evaluate the points raised in the CRL for discussion with the FDA to determine an expeditious path forward,” said Corey Fishman, Chief Executive Officer.
Iterum is a clinical-stage pharmaceutical company dedicated to the development of differentiated anti-infectives intended to combat multi-drug resistant pathogens. In doing so, the Company hopes to improve the lives of those affected by serious and life-threatening disease around the world. However, Iterum’s stock performance this month has been both a metaphorical and literal rollercoaster. Earlier this month, on July 2, 2021, Iterum’s shares plummeted 35.5% after the Company announced it had received a letter from the FDA identifying deficiencies in Iterum’s oral sulopenem. As a result, any potential approval for sulopenem had to be put on hold, which didn’t sit well with investors. Unfortunately, according to the Company’s latest press release, history has repeated itself and Iterum is now paying the price.
After reviewing Iterum’s NDA for oral sulopenem, the FDA concluded that it cannot approve the NDA in its present form. In the CRL, the FDA does acknowledge that the Phase III SURE-1 clinical trial demonstrated statistical significance regarding overall response rate of oral sulopenem compared to ciprofloxacin, an antibiotic used to treat a number of bacterial infections, in the ciprofloxacin-resistant population. For context, SURE-1 refers to Iterum’s three phase Phase III clinical trial, initiated on August 9, 2018. Aside from beginning on my birthday, the most important day of the year, the Company’s clinical trial is intended to compare Iterum’s oral sulopenem with ciprofloxacin in women with uncomplicated urinary tract infections (uUTIs).
We remain confident in the value of, and unmet medical need for, oral sulopenem to treat multi-drug resistant infections, including fast-growing quinolone non-susceptible pathogens,” continued Corey Fishman.
However, according to the provided CRL announced today, the FDA determined that additional data was necessary to support approval for the treatment of adult women with uUTIs. In the CRL, the FDA recommends that Iterum conducts at least one additional adequate and well-controlled clinical trial, potentially using a different drug comparator. Additionally, the FDA recommended that the Company performs further nonclinical investigations to determine optimal dosing regimen, however, the FDA states that this recommendation will not raise an approvability issue.
Needless to say, its back to the drawing board for Iterum…again. With that being said, its not all bad news for Iterum. According to the Company’s Q1 2021 Financial Results, Iterum was able to increase its total assets from $32,792,000 on December 31, 2021 to $115,165,000 on March 31, 2021. Iterum’s liabilities also increased marginally from $83,351,000 to $83,648,000 in the same period. Additionally, Iterum was able to increase its cash and cash equivalents to $66,640,000 at the end of Q1 2021, up from $14,508,000 at the end of the previous quarter (Q4 2020).
With this in mind, Iterum intends to review the CRL with its advisors and plans to request a Type A meeting in the coming weeks. Following the Type A meeting, anticipated for Q3 2021, Iterum expects to provide an update regarding the potential additional clinical and non-clinical work to be done prior to a resubmission of the NDA for approval of oral sulopenem.
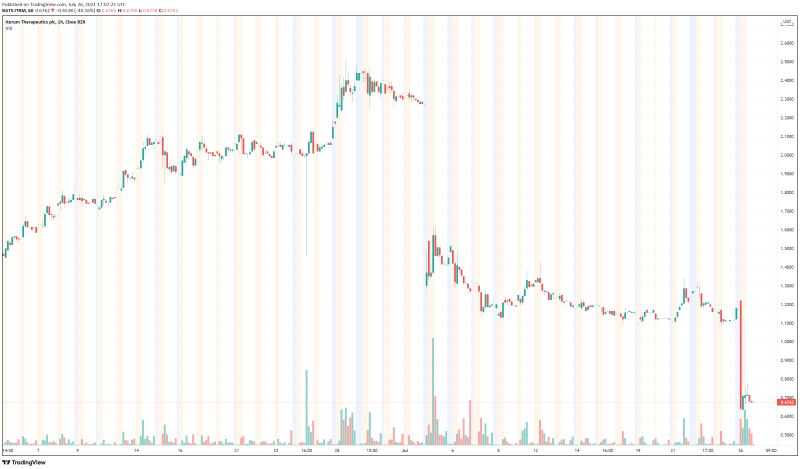
Iterum’s share price opened at $0.695, down from a previous close of $1.13. The Company’s shares are down almost 40% and are currently trading at $0.67 as of 1:08PM ET. This indicates that there has been significant change following the news.

