Acorda Therapeutics (ACOR.Q) announced today that it has entered into a distribution and supply agreement with Esteve Pharmaceuticals to commercialize INBRIJA® 33 mg in Spain.
“We are delighted to enter a partnership with ESTEVE to make INBRIJA available in Spain. This is great news for people with Parkinson’s in Spain who are in need of therapies to treat their OFF periods. ESTEVE has an impressive track record of successfully commercializing pharmaceuticals in Europe for neurological and other indications,” said Ron Cohen, M.D., President and CEO of Acorda Therapeutics.
Acorda is a biotechnology company focused on developing therapies that restore function and improve the lives of people with neurological disorders. The Company has an industry leading pipeline of novel neurological therapies addressing a range of disorders, including Parkinson’s disease, migraines and multiple sclerosis. Currently, Acorda markets two FDA-approved therapies, one of which is INBRIJA®, the Company’s levodopa inhalation powder.
INBRIJA® is a prescription medicine used in combination with carbidopa/levodopa medications to treat OFF episodes in adults with Parkinson’s. OFF episodes are periods of time when oral levodopa medication hasn’t taken effect or its effects have worn-off. During this time, individuals with Parkinson’s experience a reoccurrence of symptoms including tremors, slowness, stiffness, difficulty moving or walking, and trouble getting around. With this in mind, approximately 1 million people in the US and 1.2 million Europeans are diagnosed with Parkinson’s. Moreover, nearly 40% of the 1 million diagnosed with Parkinson’s in the US experience OFF periods.
Referring back to Acorda’s latest press release, the Company has entered into a distribution and supply agreement with Esteve to commercialize INBRIJA® in Spain. According to current population estimates, there are at least 300,000 people living with Parkinson’s in Spain, with one new case per 10,000 people developing per year. Under the terms of Acorda’s latest supply agreement, the Company will receive a significant percentage of INBRIJA®’s selling price in Spain. Furthermore, Esteve will have exclusive distribution rights to INBRIJA® in the territory.
“We are also in active discussions with additional companies for the rights to INBRIJA in other countries in Europe and the rest of the world,” continued Ron Cohen.
In a report published by ReportLinker, analysts expect the global Parkinson’s disease market to grow from $3.5 billion in 2019 to $11.5 billion in 2029, expanding at a CAGR of 12.6% during the forecast period. Looking at Acorda’s Q1 2021 financial results, the Company appears to be well positioned to capitalize on this market. As of March 31, 2021, Acorda had cash, cash equivalents, short-term investments, and restricted cash of $148.4 million compared to $102.9 million at the end of 2020. Additionally, the Company reported INBRIJA® net revenues of $5 million compared to $4.4 million year-over-year. As Acorda continues to expand commercialization of INBRIJA® globally, it may be worth keeping an eye on the Company.
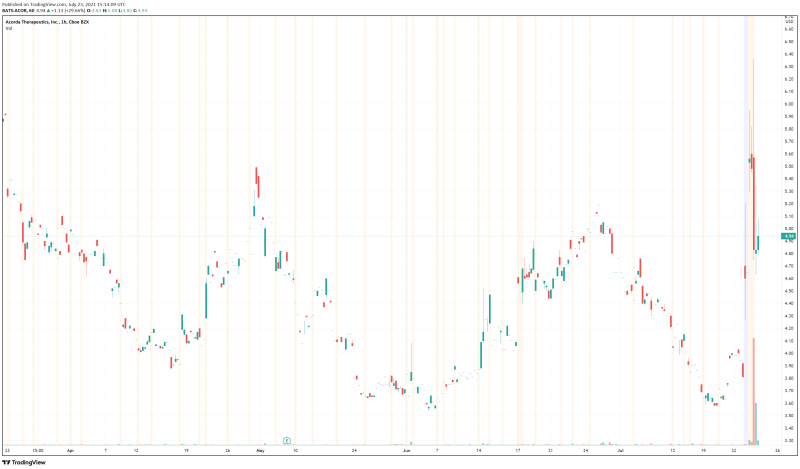
Acorda’s share price opened at $5.28, up from a previous close of $3.81. The Company’s shares are up 30% and are currently trading at $4.98 as of 11:04 AM ET. This indicates that there has been significant change following the news.

