CNS Pharmaceuticals (CNSP.Q), the company that licenses the drug candidate Berubicin to WPD Pharmaceuticals, announced today that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation for its lead investigational drug for the treatment of patients with recurrent glioblastoma multiforme (GBM).
What is Glioblastoma (GBM)?
Glioblastoma (GBM) is a fatal brain cancer that can result in death in 6 months or less if left untreated. Furthermore, glioblastomas are the most common malignant brain and central nervous system tumors, accounting for 47.7% of all cases. To make matters worse, GMB has an incidence rate of 3.21 per 100,000 population and a survival rate of 40% in the first year post-diagnosis; this number decreases significantly to 17% in the second year post-diagnosis.
Some symptoms of GBM include persistent headaches, blurred vision, vomiting, loss of appetite, changes in mood, and new onset of seizures, to name just a few. The current standard of care for newly diagnosed glioblastoma consists of maximal safe surgical resection followed by concurrent radiation therapy with temozolomide, followed by adjuvant temozolomide. In other words, surgery is performed to remove as much of the cancerous tumor as possible, followed by radiation therapy utilizing temozolomide, a medication used to treat brain tumors such as glioblastoma multiforme.
This treatment resulted in an increase median survival of 2.5 months, up from 12.1 months to 14.5 months, and an increase in 2-year survival of 26%, without a negative impact on the patients’ quality of life. However, when patients with GBM develop a recurrence of the disease, the 6-month progressive-free survival ranges between 8-15%, with a median ranging from 8-9 weeks, and overall survival extends to no more than 3-4 months.
CNS’ Potent Killer, Berubicin
Berubicin is an anthracycline, a class of anticancer agents that are among the most powerful chemotherapy drugs. Moreover, anthracyclines are effective against more types of cancer than any other class of chemotherapeutic agents. With this in mind, Berubicin is CNS’ novel therapy for the treatment of glioblastoma and is one of the first anthracyclines able to cross over the blood brain barrier (BBB) and kill tumor cells in humans. During a Phase 1 human clinical trial, Berubicin treatment of brain cancer patients appeared to demonstrate positive responses including one durable complete response.
Referring back to CNS’ latest announcement, the Company recently received Fast Track Designation for Berubicin, which will enable more frequent interactions between CNS and the FDA to expedite the development and review process for Berubicin. Furthermore, CNS recently announced the start of patient enrollment in its study of Berubicin for the treatment of recurrent glioblastoma multiforme.
According to CNS’ Q1 2021 financial results, the Company’s assets decreased from $15,853,412 on December 31, 2020 to $13,144,609 on March 31, 2021. However, CNS’ overall liabilities decreased marginally from $1,905,428 to $1,901,757 during the same period. Additionally, the Company was able to more than double its cash from $5,379,790 to $11,075,200 year-over-year. With this in mind, having received FDA approval, CNS’ Berubicin is now on the fast track towards commercialization. If successful, Berubicin will provide a much needed alternative for patients suffering with glioblastoma.
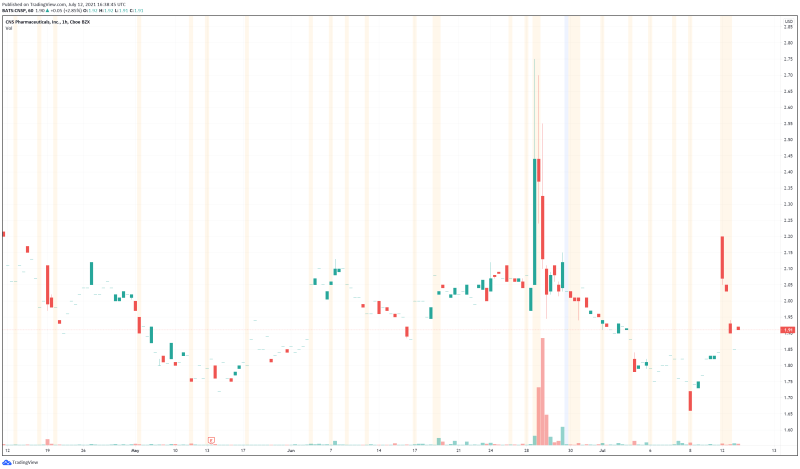
CNS’ share price opened at $1.94, up from a previous close of $1.85. The Company’s shares are up 3.24% and are currently trading at $1.91 as of 12:39PM ET. This indicates that there has been noticeable change following the news.

