Aravive (ARAV.Q), a clinical-stage oncology company developing innovative therapeutics to treat life-threatening diseases, announced positive initial results today from the Phase 1b portion of its Phase 1b/2 study of AVB-500.
“We are pleased to announce the favorable results in the first cohort of our clear cell renal cell carcinoma Phase 1b study, as we continue to advance AVB-500 and evaluate its ability to address an urgent, high unmet medical need in patients with advanced kidney cancer who have very low survival rates,” said Gail McIntyre, Ph.D., Chief Executive Officer of Aravive.
AVB-500 is a therapeutic recombinant fusion protein that has been shown to neutralize GAS6 activity by binding to GAS6. ABV-500 selectively inhibits the GAS6-AXL signalizing pathway, which is upregulated in multiple cancer types including ovarian cancer. Put simply, in pre-clinical studies, AVB-500 has show anti-tumor activity in combination with a variety of anticancer therapies, including radiation therapy, immuno-oncology agents, and chemotherapeutic drugs.
With this in mind, Aravive initiated its Phase 1b portion of the Phase 1b/2 trial of AVB-500 in March 2021. The study is intended to evaluate the tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of AVB-500 in combination with cabozantinib. Cabozantinib, commonly recognized under the brand names Cometriq and Cabometyx, is a medication used to treat various forms of cancer including medullary thyroid cancer (MTC) and hepatocellular cancer (HCC). According to Aravive, AVB-500 https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered at 15mg has generated positive results in combination with cabozantinib in patients with advanced stage kidney cancer. The data in three evaluable patients indicated that AVB-500 was well tolerated with no unexpected findings.
“We continue to focus on difficult-to-treat life threatening cancers with AVB-500, and in addition to our clear cell renal cell carcinoma clinical trial, our lead indication in paclitaxel resistant ovarian cancer is in a Phase 3 clinical trial, and we recently announced that we plan to initiate a Phase 1b/2 clinical trial in first-line metastatic pancreatic cancer in the second half of this year. We are enthusiastic about the clinical data with AVB-500 in combination with anticancer therapies that continue to show consistent PK/PD data and a favorable safety profile. These combinations may have the potential to be used in a range of different cancers,” continued Gail McIntyre.
Based on the pharmacokinetics, pharmacodynamics, and safety data of AVB-500, and approval by the Data Safety Monitoring Board (DSMB), Aravive plans to expand the dosing of 15mg of AVB-500 to an additional three patients. In doing so, the Company intends to evaluate the potential of initiating the Phase 2 portion with the same dose. As for Aravive’s current Phase 1b study, the Company will continue investigating higher doses of AVB-500 to obtain addition data.
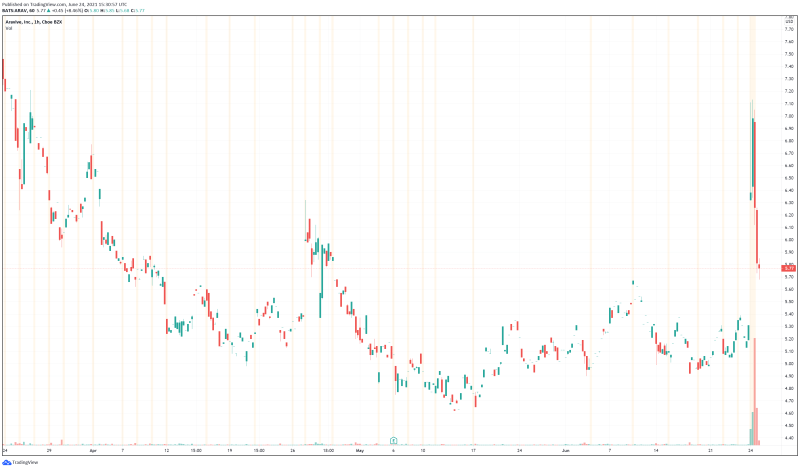
Aravive’s share price opened at $6.73 today, up from a previous close of $5.32. The Company’s shares are up 8.08% and are currently trading at $5.75 as of 11:35AM ET. This indicates that there has been noticeable change following the news.

