Tetra Bio-Pharma (TBP.T) announced today that Health Canada has accepted the Company’s New Drug Submission (NDS) for REDUVO™ and has formally entered the final review phase in the drug review process.
“There is a need for alternative therapies for patients experiencing CINV who don’t respond well to the conventional drugs used to control nausea and vomiting. Prevention of CINV remains a priority to reduce further illness in patients receiving chemotherapy. Tetra anticipates that Reduvo will be publicly and privately reimbursed by provinces and Canadian private health care plans…We will continue to work closely with Health Canada as we seek to bring this therapy to patients as soon as possible,” said chief executive officer Dr. Guy Chamberland.
For context, CINV refers to chemotherapy-induced nausea and vomiting, one of the most prominent side effects experienced by patients with cancer. Although there are numerous preventative and treatment options available for managing CINV, as many as 40% of patients with cancer still experience nausea, vomiting, or both following chemotherapy. According to a study conducted by Support Care Cancer, 71% of breast cancer patients studied experienced nausea despite being prescribed guideline-based antiemetic therapy. With this in mind, reliable and effective treatment for CINV are still few and far between.
Tetra Bio-Pharma’s REDUVO™ is a soft gel capsule intended to treat CINV as well as weight loss and severe nausea in people living with HIV. The active pharmaceutical ingredient (API) in REDUVO™ is dronabinol, also known as tetrahydrocannabinol (THC), a synthetic form of the active natural substance found in cannabis. According to Cancer Chemotherapy and Pharmacology, oral cannabinoids including dronabinol have been shown to have similar or improved efficacy when compared with conventional antiemetics used to treat CINV.
If it passes the final review phase in the drug review process, REDUVO™ will be issued a notice of compliance (NOC) as well as a drug identification number (DIN). This will permit the Company to market REDUVO™ in Canada and indicates the drug’s official approval. If REDUVO™ succeeds, it could become a mainstream treatment option for patients suffering from CINV in Canada. Tetra Bio-Pharma is definitely worth keeping an eye on as the Company inches closer to bringing REDUVO™ to the Canadian market.
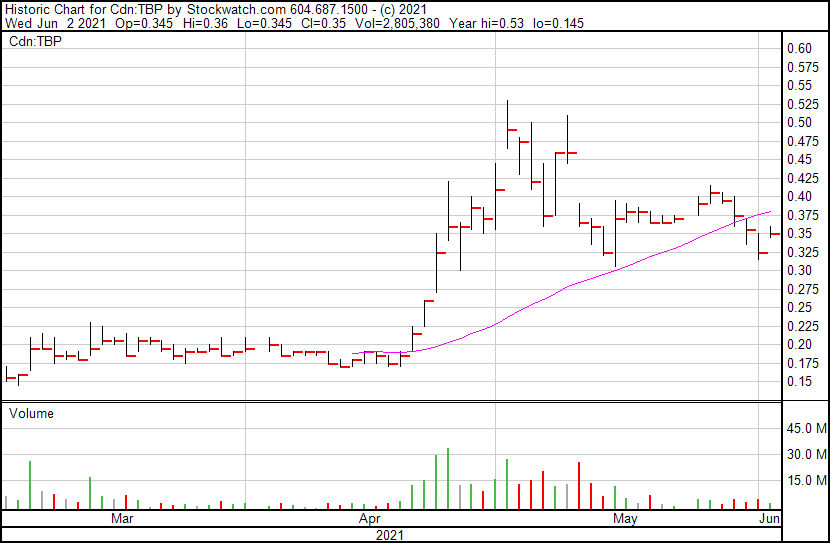
Tetra Bio-Pharma’s share price opened at $0.345, up from a previous close of $0.325. The Company’s shares are up 9.23% and are currently trading at $0.355 as of 12:40PM ET. This indicates that there has been noticeable change following the news.

