Gastroparesis
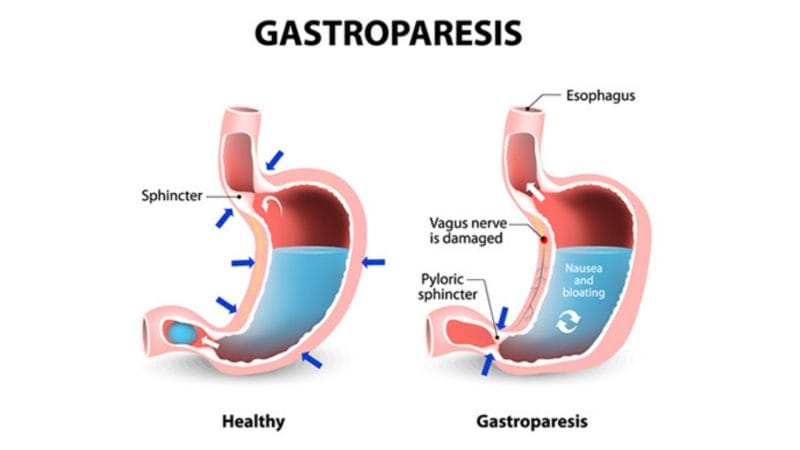
We have all felt bloated after a meal. Usually, some classic Pepto Bismol does the trick, however, some aren’t as lucky. Gastroparesis, also referred to as delayed gastric emptying, is a disorder that slows or stops the movement of food from the stomach to the small intestines. This can occur even if there is no blockage in the stomach or intestines. Diagnosis of gastroparesis includes blood tests, barium X-ray, and gastric emptying breath tests (13C-GEBT), to name a few. Some symptoms of gastroparesis include:
- feeling full shortly after starting a meal
- feeling full long after a meal
- nausea + vomiting
- heartburn or gastroesophageal reflux disease (GERD)
- bloating
With this in mind, diabetes is the most common known cause of gastroparesis. The estimated prevalence of gastroparesis in the United States (US) is approximately 6 million patients, many of which remain undiagnosed. According to a study conducted by the Journal of Neurogastroenterology and Motility, the prevalence of diagnosed gastroparesis is roughly 24 in 100,000. That being said, gastroparesis is classified as a rare medical condition according to NORD, a 501(c)(3) organization committed to the identification, treatment, and cure of rare disorders.
Treatment
Currently, the only U.S. Food and Drug Administration (FDA) approved treatment for gastroparesis is metoclopramide, a drug approved in 1979 for stomach and esophageal problems. Unfortunately, due to metoclopramide’s extensive list of severe side effects, the drug carries a black box warning. This warning limits the use of metoclopramide to no more than 3 months. With this in mind, patients are faced with limited therapeutic options.
In addition to metoclopramide, clinical guidelines suggest the off-label use of different drugs, botulinum injections, gastric stimulators, and a variety of surgical procedures, none of which sound fun. That being said, gastroparesis treatment represents a significant unmet medical need, a claim supported by the International Foundation for Gastrointestinal Disorders (IFFGD) and Gastroparesis Patient Association for Cures and Treatment Inc. (G-Pact).
Vanda Pharmaceuticals Inc.

- $654.061M Market Capitalization
Vanda Pharmaceuticals Inc. (VNDA.Q) is a global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs. The Company utilizes new technologies, including genetics and genomics, to inform drug discovery and clinical trials. To data, Vanda has two FDA-approved pharmaceuticals, namely HETLIOZ® for Non-24-Hour-Sleep-Wake-Disorder and Fanapt® for the treatment of schizophrenia in adults.
Latest News
With regards to Vanda’s clinical development pipeline, the Company has multiple products including tradipitant, VTR-297, and CFTR. In particular, Vanda’s tradipitant is a neurokinin-1 receptor antagonist that is currently in clinical development for gastroparesis and motion sickness. Most recently, on February 4, 2022, announced results from its Phase III clinical study, VP-VLY-686-3303, evaluating the efficacy and safety of tradipitant in treating symptoms of gastroparesis.
Cutting to the chase, Vanda’s clinical study did not meet its prespecified primary endpoint. For context, the study’s primary endpoint was the difference between drug and placebo on the change of severity of nausea from baseline at week 12 of treatment. However, it is worth noting that although the study did not meet its primary endpoint, both treatment arms showed significant improvements from baseline on nausea as well as other core symptoms of gastroparesis.
“While disappointed that the study did not meet its prespecified outcome, we are encouraged by the evidence that is emerging from further analysis that confirms observations made in the prior clinical study…We are committed to completing our analysis and working to bring tradipitant to patients with gastroparesis to fill a significant unmet medical need,” said Mihael H. Polymeropoulos, M.D., Vanda’s President, CEO, and Chairman of the Board.
What’s the reason? According to Vanda, the initial exploratory analysis identified potential confounders that may have masked the beneficial effect of tradipitant previously observed in the Company’s Phase II study. In layman’s terms, Vanda attributes the study’s failure to achieve its primary endpoint to factors independent of the drug itself. These factors include a baseline imbalance of rescue medication use between the two treatment arms and observed poor compliance with the study drug for some patients.
For context, compliance is the act of taking medication on schedule or taking medication as prescribed. That being said, poor compliance refers to not taking medication on schedule or as prescribed. Similarly, baseline imbalance is a term for a systematic error in a clinical trial, such that participants differ with respect to prognosis. With regards to Vanda’s clinical study, there was a notable imbalance between rescue medication use between the study’s two treatment arms.
Outcome
However, when Vanda restricted its analysis to the group of patients that used no rescue medications at baseline and adjusted for poor compliance, the Company identified strong evidence of drug effect across a number of symptoms and across the duration of the study. This includes a significant and meaningful effect at the prespecified primary endpoint of nausea change at week 12.
Additionally, Vanda’s Phase III clinical study continued to demonstrate that tradipitant is safe and well-tolerated, as seen in previous studies over the 12 weeks of treatment. In terms of adverse events, patients on tradipitant experienced a similar number of treatment-emergent adverse events as patients receiving placebo. The most common adverse event where tradipitant frequency was higher than placebo was diarrhea.
Patients that participated in the clinical program also had the opportunity to seek expanded access to tradipitant based on the benefit in the study and their individual unmet medical needs. Ten patients have received more than 3 months of tradipitant treatment, 6 of whom have received at least 1 year of tradipitant treatment. Nonetheless, Vanda’s stock has plummeted more than 20% following the Company’s latest news, as is usually the case when a clinical trial fails to meet its primary endpoint.
Financials
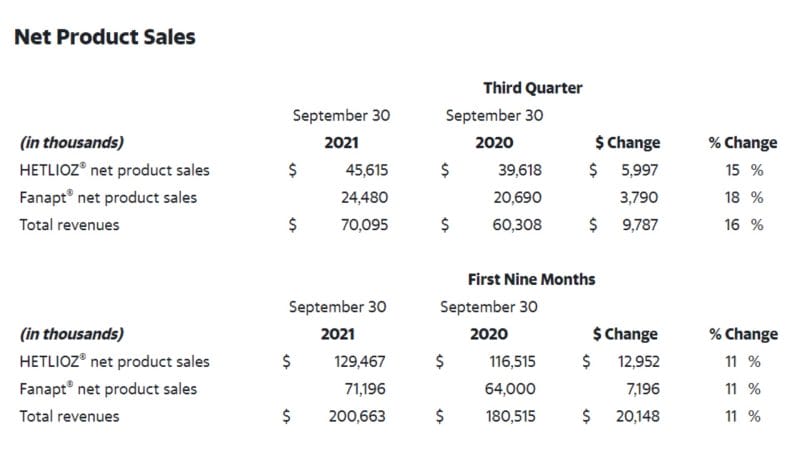
Historically, with two marketed pharmaceuticals under its belt, Vanda has performed quite well. According to the Company’s Q3 2021 Financial Results, total net product sales from HETLIOZ® and Fanapt® were $70.1 million, representing a 16% increase compared to $60.3 million in Q3 2020. For the nine months ended Q3 2021, these numbers translate to $200.7 million, representing an 11% increase compared to $180.5 million in the first nine months of 2020.
Furthermore, Vanda’s income before taxes was $10.7 million in Q3 2021 compared to $8.4 million in Q3 2020. For the first nine months of 2021, the Company’s income before taxes was $33.8 million compared to the same period in 2020. Overall, Vanda’s cash and cash equivalents were $406 million as of September 30, 2021, representing an increase of $57.4 million compared to September 30, 2020.
Moving forward, Vanda plans to continue the analysis of the Company’s Phase III clinical study data and prepare the results for submission to peer review journals and to the regulatory authorities. Furthermore, Vanda intends to schedule a conference call to discuss the Company’s results in the coming weeks. As for Vanda’s tradipitant, the FDA has imposed a partial clinical hold on tradipitant clinical protocols of longer than 12 weeks duration.
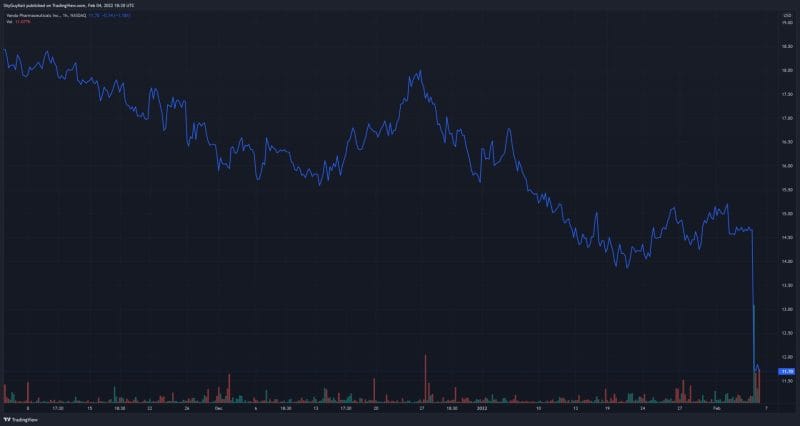
Vanda’s share price opened at $11.34, down from a previous close of $14.65. The Company’s shares are down -19.83% and were trading at $11.74 as of 1:29 PM EST.

