
Spruce Biosciences Inc. (SPRB.Q) is a late-stage biopharmaceutical company developing and commercializing novel therapies for rare endocrine disorders with significant unmet medical needs. The Company is credited for developing its wholly-owned product candidate, tildacerfont, a CRF1 receptor antagonist. Endocrine disorders are medical conditions where the endocrine system, which produces hormones in our body, does not function properly.
To be more specific, the endocrine system is a collection of glands and organs that regulate bodily processes through hormones. Primary parts of the endocrine system include the hypothalamus, thyroid, and pancreas, among others. With this in mind, there are a variety of potential causes for endocrine disorders, including tumors, genetic factors, or hormonal imbalances. Common endocrine disorders include:
- Diabetes – a condition causing high blood glucose levels
- Hyperthyroidism – occurs when the thyroid gland produces too many hormones
- Hyp0thyroidism – occurs when the thyroid gland does not produce enough hormones
- Cushing’s Syndrome – occurs when there is an excess of the hormone cortisol, which helps the body respond to stress
- Congenital Adrenal Hyperplasia – an inherited defect in which the adrenal glands’ ability to produce cortisol is impaired
- impaired cortisol synthesis leads to chronic elevations of adrenocorticotropic hormone
Referring back to Spruce’s tildacerfont, the Company’s product candidate is designed to potentially offer markedly improved disease control and reduced steroid burden in patients with classic congenital adrenal hyperplasia (CAH) and other diseases characterized by elevated levels of adrenocorticotropic hormone (ACTH). That being said, tildacerfont binds CRF1 receptors to the pituitary gland, inhibiting the excessive production of ACTH.
Latest News

That’s the SparkNotes version of it. Spruce’s tildacerfont is currently in late-stage trials in adult patients with CAH, with a recently initiated Phase 2 trial in pediatric classic CAH. Most recently, on January 24, 2022, Spruce provided an update on its clinical programs, upcoming milestones, and strategic priorities for enhancing the design and accelerating patient recruiting into the CAHmelia studies, which are evaluating tildacerfont for the treatment of adult classic CAH.
“Following a comprehensive assessment of the CAHmelia program, we’ve identified opportunities to accelerate patient recruitment and enhance the designs of the studies evaluating the potential of tildacerfont as a treatment for adult patients with classic CAH,” said Javier Szwarcberg, M.D., MPH, Chief Executive Officer of Spruce Biosciences.
Before I try and navigate Spruce’s maze of a press release, let me outline tildacerfont’s indications. Currently, the Company tildacerfont is indicated for adult classic CAH, pediatric classic CAH, and polycystic ovary syndrome (PCOS), a hormonal disorder common among women of reproductive age. Lucky for me, Spruce has provided an update for each of the aforementioned indications. Sarcasm.
Regarding Spruce’s Late-Stage CAHmelia Program in Adult Classic CAH, the Company announced that it intends to significantly expand the number of study sites for its CAHmelia-203 and CAHmelia-204 studies evaluating tildacerfont. To be more specific, Spruce intends to significantly expand the number of study sites by up to 50 new sites, for a total of up to 130 sites worldwide. This includes adding sites to currently selected regions in the United States (US), Canada, and Germany, to name just a few.
Amended Protocols for CAHmelia-203 & CAHmelia-204

Moving forward, Spruce also plans to identify new sites within additional countries. The addition of new sites is expected to expand recruitment capabilities and ultimately accelerate enrollment. In Spruce’s latest press release, the Company also provided protocol amendments to its CAHmelia-204 and CAHmelia-203 studies. While I could try and break down each one, we would be here all day. I’m also not a scientist. Instead, let me summarize.
Spruce is implementing two key protocol changes to its CAHmelia-204 study, including amending the androstenedione (A4) inclusion criteria and eliminating the glucocorticoid conversion requirement. If that doesn’t make sense, good. All you need to know is that this is expected to increase enrollment into the Company’s CAHmelia-204 study. Next.
Regarding Spruce’s next amendment, the Company is amending the primary endpoint in its CAHmelia-204 study and adjusting the A4 and ACTH inclusion criteria in CAHmelia-203. From what my small brain can comprehend, the Company’s amendment to the primary endpoint in the CAHmelia-204 study will make the study’s primary endpoint a key secondary endpoint instead.
As for Spruce’s adjustment of A4 and ACTH inclusion criteria in CAHmelia-203, that one’s a bit easier to explain. In the Company’s words, it will be increasing the A4 inclusion criterion to 2.5 times the Upper Limit of Normal (ULN) and is removing the ACTH inclusion criterion. Put simply, Spruce has determined that the A4 level inclusion criterion alone provides sufficient evidence of excessive adrenal stimulation by ACTH. As a result, the Company is removing the ACTH inclusion criteria.
Additionally, Spruce announced that it will be implementing an optional pre-screening protocol to enable prompt determination of key inclusion criteria under the Company’s revised CAHmelia-203 and CAHmelia-204 study protocols. This is expected to increase overall screening and improve assessment efficiency of eligibility by study sites into either CAHmelia-203 or CAHmelia-204.
Pediatric Classic CAH Program

Spruce recently initiated a Phase 2 clinical trial investigating tildacerfont for the treatment of classic CAH in children. As expressed by the Company, there is a significant medical need to bring androgen-lowering and glucocorticoid-sparing therapies to pediatric classic CAH patients. For context, glucocorticoids drugs are commonly used for the treatment of CAH. However, glucocorticoids in children can result in adverse effects including stunted growth and premature puberty.
With this in mind, Spruce also announced that the Pediatric Committee (PDCO) of the European Medicines Agency (EMA) adopted a positive opinion on its agreement with the proposed Pediatric Investigational Plan (PIP) of tildacerfont for the treatment of CAH. PDCO’s opinion endorsed the clinical program to evaluate the safety, tolerability, and efficacy of tildacerfont for the treatment of CAH in patients from one year to age to less than 18 years of age.
Furthermore, PDCO has also granted a waiver for the treatment of CAH in patients less than one year of age. That being said, the adoption of the PIP will assist Spruce in initiating a Phase 3 registrations program in pediatric classic CAH following the successful completion of the Company’s current Phase 2 clinical trial investigating tildacerfont for the treatment of classic CAH in children.
PCOS Program + Financial Update
Finally, Spruce also announced that it has recently initiated a randomized placebo-controlled dose-escalation study evaluating the safety and efficacy of tildacerfont in subjects with polycystic ovary syndrome (PCOS). Ultimately, by reducing ACTH-stimulated adrenal androgen production, the Company believes tildacerfont has the potential to treat the potential onset of PCOS.
“By increasing the number of global trial sites and effecting protocol amendments, we will be well-positioned to meet our revised topline data milestones. In addition, we have reprioritized activities which have enabled us to extend our anticipated cash runway by approximately 6 months, taking us into Q2 2024,” concluded Javier Szwarcberg.
To conclude its press release, Spruce announced that the Company estimates that its unaudited cash, cash equivalents, and investments were $121.4 million as of December 31, 2021. Furthermore, Spruce’s strategic prioritization of activities has resulted in projected program cost reductions and deferrals of expenditures that are aligned with its updated program timelines.
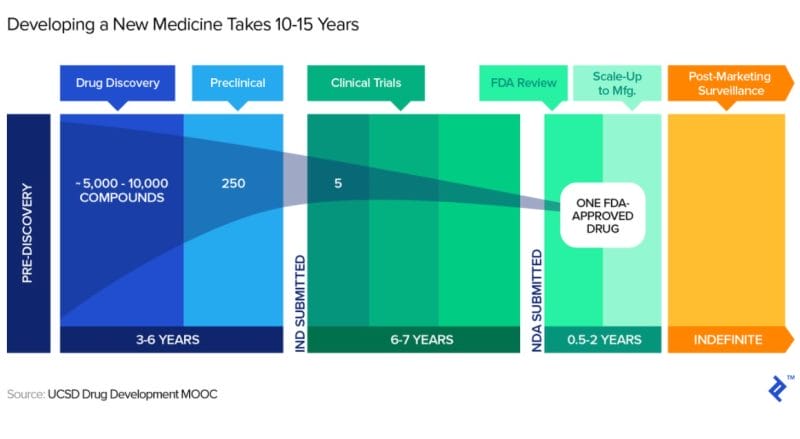
It is also worth noting that Spruce has extended its expected cash runway by approximately 6 months, from Q4 2023 to Q2 2024. For context, a cash runway refers to the time a company can operate at a loss before it runs out of money. That being said, expecting any biotech company to be profitable from the get-go is wishful thinking. Typically, the timeline for a new drug from submission of an Investigational New Drug application to market entry, post regulatory approval, is roughly eight years.
Looking forward, Spruce anticipates the following milestones:
- Completion of enrollment from the Phase 2 proof of concept clinical trial in polycystic ovary syndrome (PCOS) by the end of 2022 and topline results by the first half of 2023
- Topline results from CAHmelia-203 in adult classic CAH patients with poor disease control by the second half of 2023
- Topline results from CAHmelia-204 in adult classic CAH patients with good disease control by the second half of 2024
If you have read the entirety of this article then you are either a masochist or really enjoy biotech. Either way, Spruce has its foot in a niche market that is estimated to expand at a compound annual growth rate (CAGR) of 7.1% from 2017 to 2023. However, Spruce has fallen quite far off the pedestal it once stood on. According to the Company’s 52-week range, Spruce’s share price has plummeted from $28.49 to $2.25. Why? You’ll have to stay tuned to find out.
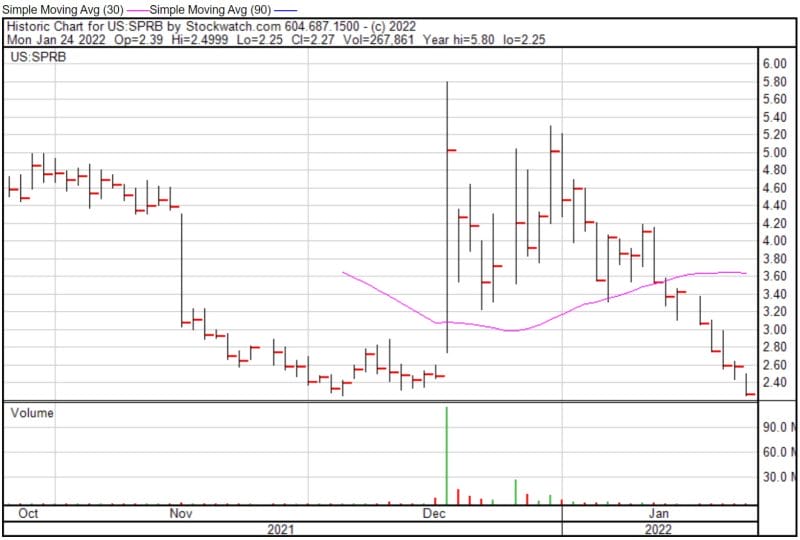
Spruce’s share price opened at $2.40 today, down from a previous close of $2.58. The Company’s shares are down -12.3372% and were trading at $2.2617 as of 12:35 PM EST.

