Latest News

Revive Therapeutics Ltd. (RVV.C) has provided an update for the Company’s U.S. Food and Drug Administration (FDA) Phase 3 clinical trial to evaluate the safety and efficacy of Bucillamine in patients with mild to moderate COVID-19. For context, Revive is a life sciences company focused on the research and development of therapeutics for medical needs and rare disorders. The Company has a variety of products currently under development including, Psilocybin for Methamphetamine Use Disorder, TBI/Stroke, Depression, and Anxiety. Additionally, Revive is also exploring Cannabidiol CBD for diseases such as Autoimmune Hepatitis.
As for the Company’s focus on Bucillamine in the treatment of infectious diseases like COVID-19, Revive is well-positioned to capitalize on the global coronavirus current therapy market. Keep in mind, this profitable market is expected to grow from $14.63 billion in 2020 to $16.18 billion in 2021, expanding at a compound annual growth rate (CAGR) of 14.8%. By 2025, this market is projected to reach an impressive valuation of $35.42 billion at a CAGR of 20%. Let’s rewind a bit. Following the successful closing of a $23 million bought deal financing on February 12, 2021, Revive announced that it would aggressively expand from 14 clinical sites to up to 50 clinical sites in order to meet its next enrollment goals for its Phase 3 clinical trial. With this in mind, the Company has currently engaged a total of 46 clinical sites in fourteen states, including Alabama, Arkansas, and California, to name just a few.
The Clinical Study
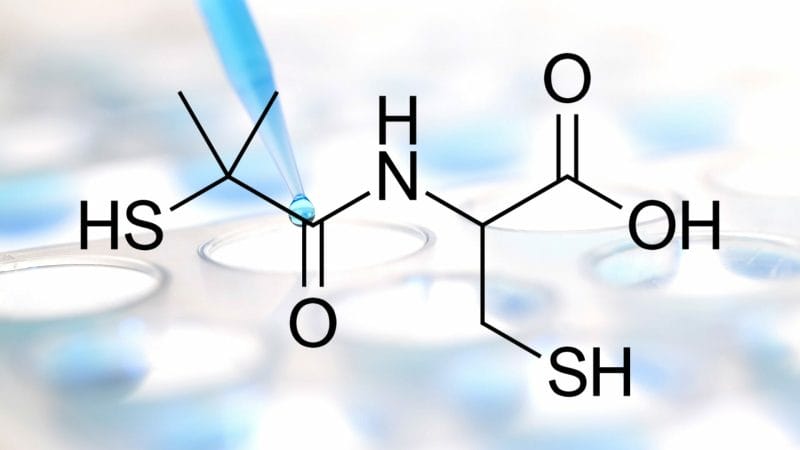
Revive’s Phase 3 confirmatory clinical trial titled, “A Multi-Center, Randomized, Double-Blind, Placebo-Controlled Study of Bucillamine in Patients with Mild-Moderate COVID-19,” will enroll up to 1,000 patients that will be randomized to Bucillamine or placebo for up to 14 days. By now you’re probably wondering what Bucillamine is, so let me give you a crash course. Bucillamine is an antirheumatic agent with a well-known safety profile and has been prescribed in the treatment of rheumatoid arthritis in Japan and South Korea for more than 30 years.
For context, antirheumatic drugs refer to agents used in the therapy of inflammatory arthritis. Okay, but what the hell does that have to do with COVID-19? Well, COVID-19 is a contagious disease caused by severe acute respiratory syndrome coronavirus 2, also known as SARS-CoV-2. With this in mind, several antirheumatic disease therapies have emerged as potential treatments for COVID-19. More specifically, Bucillamine has been shown to decrease the binding of SARS-CoV-2 spike protein to its receptor, thereby decreasing the virus’ entry efficiency and inhibiting its live virus infection.
Compared to traditional antirheumatics, Bucillamine is 16x more potent than N-acetylcysteine (NAC), which has been shown to prevent acute lung injury caused by the influenza virus in the past. More recently, NAC has been used to treat COVID-19 patients due to its antioxidant, anti-inflammatory, and immune-modulating characteristics. In addition to being 16x more potent, Bucillamine has demonstrated greater potential in preventing acute lung injury during influenza infection. Referring back to Revive, on July 31, 2020, the Company received approval from the FDA to conduct its Phase 3 clinical trial for Bucillamine. The primary objective of this study is to compare the frequency of hospitalization or death in patients with mild to moderate COVID-19 receiving Bucillamine therapy to those receiving placebo. The study’s Primary Endpoint is as follows:
- The proportion of patients meeting a composite endpoint of hospitalization or death from the time of the first dose through Day 28 following randomization
- Efficacy will be assessed by comparing clinical outcomes, disease severity, supplemental oxygen use, and progression of COVID-19 between patients receiving standard-of-care plus Bucillamine and patients receiving standard-of-care plus placebo
- Safety will be assessed by reported pre-treatment adverse events and treatment-emergent adverse events, laboratory values, vital signs, and peripheral oxygen saturation.
- The Independent Data and Safety Monitoring Board (DSMB) will actively monitor interim data for the ongoing safety of patients and will recommend continuation, stopping, or changes to the conduct of the study based on the interim analysis reports
The Point
Getting to the point, the DSMB supported the continuation of the clinical study in its last meeting seeing as there were no serious adverse events or safety concerns reported. The final interim analysis meeting, which will occur at 800 completed patients, is expected to be held in Q4 2021. With this in mind, in the event that the results provided are sufficient, the Company plans to eventually file an Emergency Use Authorization (EUA) with the FDA for Bucillamine to treat mild to moderate COVID-19. Furthermore, Revive is in discussions with its manufacturing partner to secure a commercial supply of at least 5 billion Bucillamine tablets to potentially treat 50 million people globally for 2022.
“As we move forward in our Phase 3 study in COVID-19 with the aim to seek EUA approval from the FDA for Bucillamine in the treatment of mild to moderate COVID-19, we are also cognizant of the rapidly changing landscape of COVID-19 specifically with the Delta variant becoming widespread. The incorporation of adding viral load testing to patients in the Study, along with our support in the research of the potential utility of thiol-based drugs, like Bucillamine, in the Delta variant of COVID-19, shows our confidence in Bucillamine’s potential as a safe and effective oral treatment for mild to moderate COVID-19. We recognize the market opportunity for Bucillamine and we are in discussions with our manufacturing partners to ensure that billions of Bucillamine tablets can be made available in 2022 to support our future commercialization partners and the millions of people globally,” said Michael Frank, CEO of Revive Therapeutics.
It is worth noting that Revive has decided to incorporate viral load testing to a minimum of 300 patients in its Phase 3 clinical study. This will enable the Company to quantify the speed at which Bucillamine can reduce viral infection of patients throughout the course of treatment. As a result, this will allow Revive to understand and determine the most optimal time to introduce Bucillamine in the treatment course.
Financials
According to Revive’s Annual Financial Statements, the Company had cash and cash equivalents of CAD$16,599,663 on June 30, 2021, compared to CAD$1,381,483 year-over-year (YOY). In the same period, Revive’s total assets and total liabilities increased to CAD$29,806,400 and CAD$597,533, respectively. For the year ended June 30, 2021, the Company reported a comprehensive loss of CAD$20,118,802. To date, Revive has not earned revenue and has accumulated a deficit of CAD$36,439,850 with a working capital of CAD$16,197,128 as of June 30, 2021. Currently, the Company has approximately 317.06 million shares outstanding, a market capitalization of $149.02 million, and an average trade volume of 484,721. Keep in mind, Revive has traded 468,262 shares today so far.
Looking forward, Revive is continuing discussions with reputable international pharmaceutical companies seeking to obtain commercial rights to Bucillamine as a treatment for COVID-19 in various regions, including Europe and Asia. In light of these discussions, the Company is pursuing a commercialization plan that would leverage the clinical results generated from the U.S. Phase 3 study, which would allow for drug approvals globally. Additionally. Revive has several milestones planned for Q4 2021:
- a Phase 2 study for Psilocybin in Methamphetamine Use Disorder at the University of Wisconsin
- Initiation of Phase 2 clinical study of CBD in the treatment of Autoimmune Hepatitis
- FDA pre-IND meeting for Psilocybin oral thin film
With this in mind, Revive has a diverse portfolio of products targeting a variety of addressable markets, including mental health, neurological, substance use disorder, liver diseases, and infectious diseases. While most of Revive’s products are in their pre-clinical stage of development, the Company’s Bucillamine is on track to achieve commercialization in 2022. Ultimately, armed with its Bucillamine, Revive is well-positioned to capitalize on the rapidly expanding global coronavirus current therapy market.

Revive’s share price opened at $0.45 and is currently up 4.44%, trading at $0.47 as of 12:21 PM ET.