Acasti Pharma (ACST.V) announced today that it has initiated its planned pharmacokinetic (PK) bridging study to evaluate the bioavailability of intravenous (IV) GTX-104 compared to oral nimodipine capsules in 50 healthy subjects.
“We believe GTX-104 has the potential to provide improved bioavailability and lower intra-subject variability compared to oral capsules, which could translate into better blood pressure control in these critically ill patients. Moreover, it could provide a more convenient dosing schedule that would be easier to https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngister to patients who are unconscious,” commented Jan D’Alvise, Chief Executive Officer of Acasti.
Acasti Pharma

Acasti Pharma is a late-stage pharma company with drug delivery capability and technologies addressing rare and orphan diseases. With this in mind, the Company’s drug delivery technologies are intended to provide superior onset of action, enhanced efficacy, reduced side effects, and more convenient drug delivery when compared to currently marketed drugs. Some of the underserved orphan diseases Acasti is targeting include Subarachnoid Hemorrhage (SAH), Ataxia Telangiectasia (A-T), and Postherpetic Neuralgia (PHN). To date, GTX-104, GTX-102, and GTX-101, the Company’s three lead clinical assets, have all been granted Orphan Drug Designation (ODD) by the FDA. Having received ODD, all three assets will receive seven years of marketing exclusivity upon launch in the United States and are protected by over 40 granted and pending patents.
It is worth noting that the FDA doesn’t give ODD to every Tom, Dick, and Harry. Instead, ODD is intended to help advance the evaluation and development of products that demonstrate promise for the diagnosis and treatment of rare diseases or conditions. Through various incentives, ODD enables sponsor companies like Acasti to address the hefty costs associated with the research, development, and approval of a product. In addition to seven years of market exclusivity, some other incentives include a 25% federal tax credit for expenses related to clinical research within the US, the ability to qualify and compete for research grants, and the waiver of Prescription Drug User Fee Acr (PDUFA) fees for orphan drugs, which was valued at roughly $2.9 million in 2021.
SAH & GTX-104
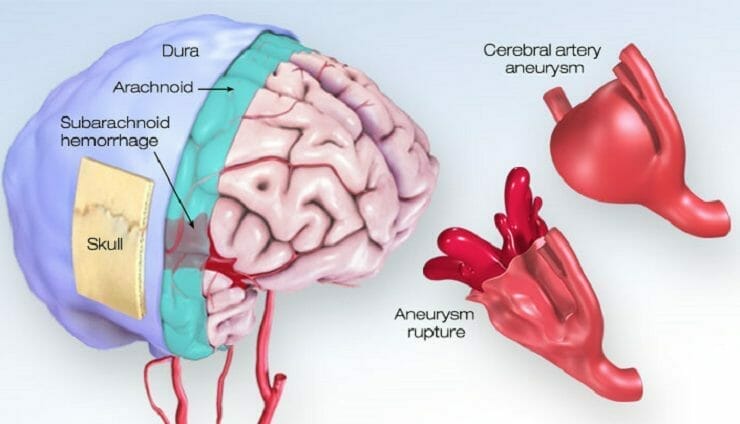
As previously mentioned, Acasti announced today that it has initiated its PK bridging study to evaluate the bioavailability of IV GTX-104 compared to oral nimodipine in 50 healthy subjects. GTX-104 is the Company’s clinical-stage, nanoparticle formulation of nimodipine being developed for infusion in SAH patients. For context, SAH refers to the bleeding the occurs over the surface of the brain in the space between the brain and the skull. Keep in mind, this area contains blood vessels that supply the brain. SAH commonly occurs following the rupture of an aneurysm, resulting in a relatively uncommon type of stroke. In total, this type of stroke accounts for roughly 5% of all strokes and has an incidence of six per 100,000 person-years. In other words, SAH-related strokes would affect six persons out of 100,000 over the course of a year.
“SAH is a devastating condition that afflicts more than 50,000 patients per year in the United States and is one of the most expensive acute conditions to treat. As a result, we believe GTX-104, through our unique formulation, has the potential to address a sizable market opportunity with a significant unmet medical need,” continued D’Alvise.
The buildup of blood over the surface of the brain as a result of SAH can also result in brain cell damage, life-long complications, and disabilities. Some of the immediate symptoms associated with SAH include loss of consciousness, nausea, seizures, trouble speaking, and confusion, to name just a few. Although SAH isn’t necessarily common, it often occurs at a relatively young age, mostly affecting patients under the age of 60. In particular, approximately 10-15% of aneurysmal SAH (aSAH) patients younger than 45 die before reaching the hospital. Overall, an estimated 70% of aSAH patients experience death or dependence, and 50% die within one month after hemorrhaging. Some immediate symptoms of aSAH include pain surrounding the eye, weakness or numbness on one side of the body, dilated pupils, and more.
Referring back to GTX-104, Acasti expects to receive results from its PK bridging study in the first half of 2022. The primary objective of this study is to evaluate the relative bioavailability of GTX-104, and the secondary objective is to assess the safety and tolerability of GTX-104, compared to nimodipine oral capsules. It is worth noting that nimodipine is the current standard of care used to treat SAH. Per the study protocol, subjects will randomly be assigned in a 1:1 ratio to one of two treatment sequences, AB or BA. Patients first assigned to Treatment A will receive GTX-104 https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered via infusion over 72 hours. On the other hand, patients first assigned to Treatment B will be https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered two nimodipine capsules, 30mg each, every 4 hours for 72 hours.
As reaffirmed in a commentary received by Equity Guru from Acasti’s communications partner JQA Partners Inc., GTX-104 is an intravenous (IV) formulation of nimodipine, thereby providing improved precision in dosing. To build on this point, intravenous drug https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration typically has a bioavailability of 100% in addition to having a rapid onset of action time. Compared to other methods of drug https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration, including intramuscular, oral, and cutaneous, IV https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistration is the best way to deliver a precise dose efficiently and in a well-controlled manner. With this in mind, Acasti’s PK study is the next required step in the Company’s proposed 505(b)(2) regulatory pathway for GTX-104.
The 505(b)(2) pathway provides manufacturers with an opportunity to acquire FDA approval without jumping through all of the hoops associated with a New Drug Application (NDA). Drug formulations like Acasti’s GTX-104 that aren’t necessarily new novel molecular entities but provide new aspects related to indication, dosage form, regimen, and more, are potentially eligible for the 505(b)(2) pathway. If the Company is able to successfully establish a regulatory pathway for GTX-104, Acasti will be able to submit its drug formulation for FDA review. Following a review with the FDA, Acasti intends to finalize the design of the Company’s Phase 3 safety study for GTX-104 in SAH patients. This study is expected to commence in the second half of 2022.
Financials
According to Acasti’s Q1 2022 results for the quarter ended June 30, 2021, the Company had cash and cash equivalents of USD$40,975,000 compared to USD$50,942,000 on March 31, 2021. The Company’s total assets and total liabilities increased to USD$60,453,000 and USD$6,996,000 in the same period, respectively. However, Acasti’s reported net loss decreased from USD$4.7 million on June 30, 2020, to USD$3.1 million on June 30, 2021. In the same period, the Company’s loss from operating activities decreased to USD$3.1 million compared to USD$4.1 million due to a reduction in research and development, general https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistrative expenses, and sales and marketing expenses. With this in mind, GTX-104 looks like a promising drug formulation for an addressable market with an estimated value of $300 million.
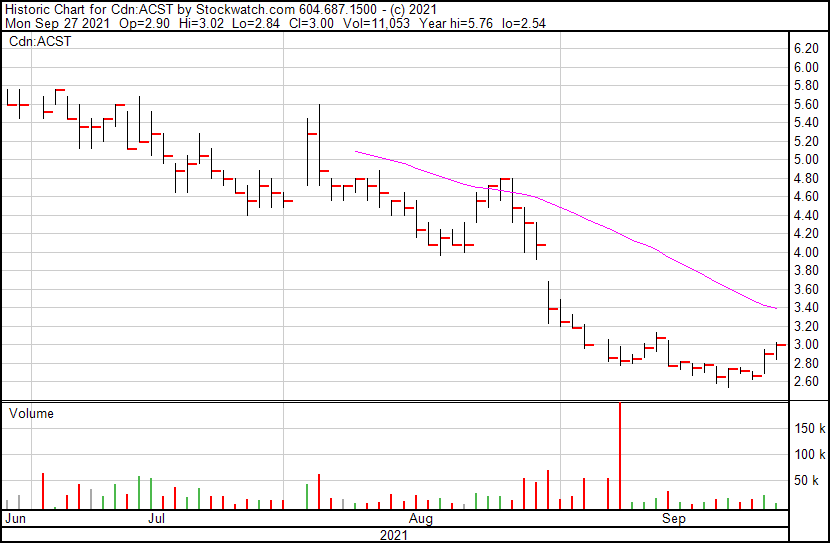
Acasti’s share price opened at $2.90 and is currently up 4.14%. The Company’s shares were trading at $3.02 following the news as of 12:01 PM ET.

