Innate Pharma S.A. (IPHA.Q) announced today that AstraZeneca has presented results from the randomized COAST Phase 2 trial during the European Society for Medical Oncology (ESMO) Congress 2021 on September 17, 2021.
“We’re pleased to see the monalizumab COAST results, particularly the improved clinical outcomes for patients with unresectable, Stage III non-small cell lung cancer,” said Mondher Mahjoubi, Chief Executive Officer of Innate Pharma.
Innate Pharma is a global, clinical-stage oncology-focused biotech company committed to improving treatment and clinical outcomes for patients through therapeutic antibodies that harness the immune system to fight cancer. With this in mind, monalizumab is a potentially first-in-class immune checkpoint inhibitor targeting NKG2A receptors expressed on tumor-infiltrating cytotoxic CD8+ T cells and NK cells. Put simply, monalizumab is intended to re-establish a broad anti-tumor response. So, what does AstraZeneca have to do with any of this? In April 2015, Innate Pharma signed a co-development and commercialization agreement with AstraZeneca to accelerate and broaden the development of monalizumab. However, in 2018, AstraZeneca obtained full oncology rights to monalizumab. With that being said, the ongoing development for monalizumab is focused on investigating the checkpoint inhibitor in various combination strategies in different malignancies.
Moving on, COAST is a Phase 2 trial being conducted by AstraZeneca in 82 centers across nine countries in North America, Europe and Asia. This Phase 2 trial is intended to investigate durvalumab, an FDA-approved immunotherapy for cancer, alone or in combination with either monalizumab or oleclumab. For context, oleclumab is a drug that was designed for the treatment of various cancers, including pancreatic and colorectal cancers. The primary endpoint of the COAST Phase 2 study is Objective Response Rate (ORR) as a measure of anti-tumor activity. Secondary endpoints include safety, duration of response, overall survival, and progression-free survival (PFS).
“Monalizumab is one of the first checkpoint inhibitors targeting a NK cell receptor, and today’s results further support the role it can play in treating certain cancers by blocking the inhibitory receptor, NKG2A. We look forward to continuing to invest in NK cell science and further advancing the next wave of scientific innovation at Innate,” continued Mondher Mahjoubi.
Stage III NSCLC:
NSCLC refers to non-small cell lung cancer. With this in mind, lung cancer is split into NSCLC and small cell lung cancer, however, 80-85% of cases are classified as NSCLC. Stage III NSCLC indicates that cancer has locally advanced and spread to the lymph nodes on the same side of the chest as the primary lung tumor. Stage III NSCLC is further divided into three subcategories, including IIIA, IIIB, and IIIC, indicating how much the cancer has spread locally.
With the formalities out of the way, let’s talk about the meat of Innate Pharma’s latest press release, which caused the Company’s shares to climb more than 80% today. According to results from AstraZeneca’s COAST Phase 2 trial, the interim analysis showed monalizumab in combination with durvalumab improved PFS and ORR compared to durvalumab alone in patients with Stage III NSCLC. In other words, monalizumab has achieved both its primary and secondary endpoints, marking a significant milestone for the Company.
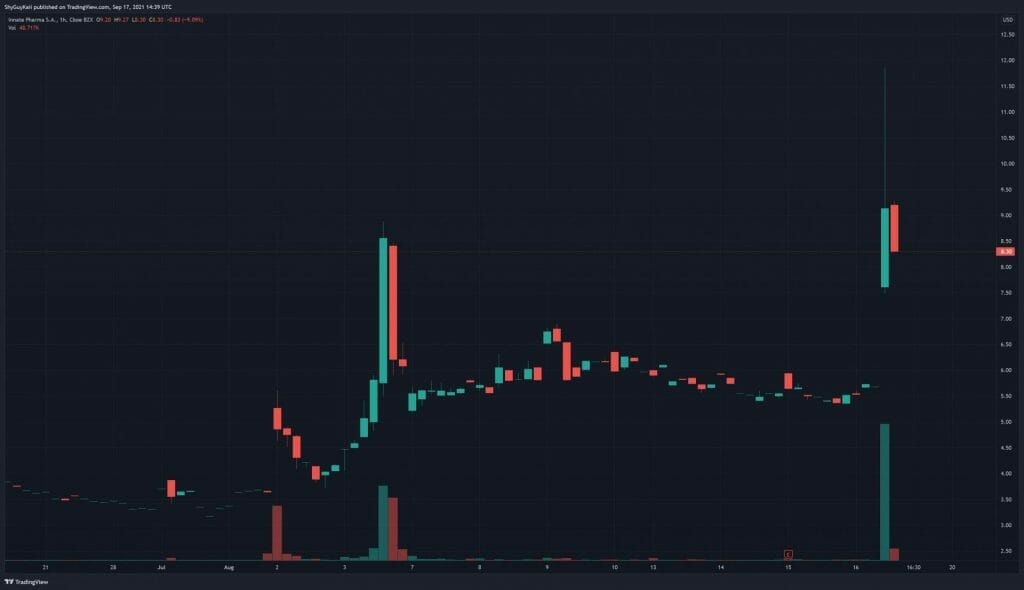
Innate Pharma’s share price opened at $7.60 today, up from a previous close of $5.75. The Company’s shares are up 45% and are currently trading at $8.34 as of 10:41 AM ET.

