Braxia Scientific (BRAX.C) announced they will be conducting a randomized clinical trial that will involve giving patients multiple doses of psilocybin.
Braxia says this is Canada’s first psilocybin trial where patients will be receiving multiple doses of psilocybin. The trials will be conducted at the Canadian Rapid Treatment Centre of Excellence (CRTCE), a wholly-owned subsidiary of Braxia.
The trial is aimed at fighting treatment-resistant depression (TRD), and some participants enrolled in the study will have previously tried electroconvulsive therapy and/or intravenous ketamine in order to fight TRD.
“This will be the broadest study of its kind,” stated Dr. Joshua Rosenblat, director of CRTCE. “Whereas most other treatment-resistant depression (TRD) studies limit participation to patients that have not found relief from a maximum of five other potential remedies, our research will not have an upper limit, and will even include patients that have endured dozens of unsuccessful medical trials, including ketamine and electroconvulsive therapy. By including everyone with more than two failed medical trials, we are increasing the degree to which the results can be applied to a larger population, making our findings much stronger. Furthermore, we will have less exclusion criteria and are even including patients with bipolar depression – a huge first for the field – or comorbid disorders, which were excluded in psilocybin studies done by other companies.”
CRTCE already operates functional ketamine treatment centers, and so they have an existing pool of patients to draw the candidates for this upcoming psilocybin trial. They currently run clinics in Toronto, Mississauga and Ottawa.
By researching how multiple doses of psilocybin affects patient outcomes, Braxia is contributing valuable information to the debate over how regularly psilocybin doses will be needed or how useful receiving multiple psilocybin treatments will be. Is psilocybin therapy just something you do once? If not, how often should you do it? If it doesn’t work the first time, do more trips help fight depression? These are all important questions, and Braxia’s clinical trial may help produce an answer.
Additionally, increasing their psilocybin footprint will help Braxia diversify, as many of their current treatments involve ketamine.
“Integrating psilocybin provides immense opportunity for benefit for those dealing with treatment-resistant depression. Unfortunately, over one-third of the more than 300 million people suffering with depression worldwide fail to adequately respond to currently approved treatments, and thus the TRD market is very large and disproportionately dominates the majority of mental health services,” added Braxia Scientific CEO Dr. Roger McIntyre.
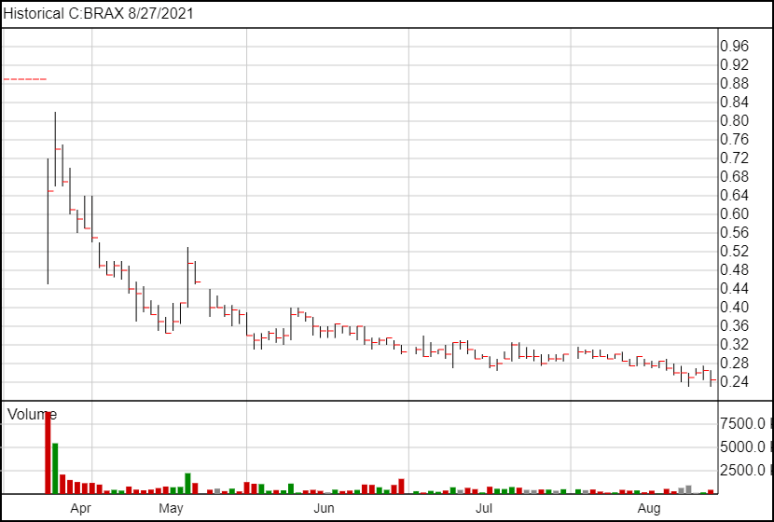
Following today’s news, Braxia shares are down 2 and a half cents and are currently trading at 24 cents.