Vivos Therapeutics (VVOS.Q) announced today that the U.S. Food and Drug Administration (FDA) has granted 510(k) market clearance to Vivos’ mmRNA (modified mandibular Repositioning Nighttime Appliance) device, not to be confused with mRNA vaccines, for treating mild to moderate obstructive sleep apnea (OSA), sleep-disordered breathing and snoring in adults.
“The FDA’s market clearance of Vivos’ newest device, the mmRNA appliance, represents a significant milestone in our ongoing efforts to provide the best possible treatment for people who continue to suffer needlessly from OSA, a debilitating condition that causes or contributes to a wide range of chronic health issues,” said Kirk Huntsman, Vivos Chairman and CEO.
As someone who can’t remember the last time I had a good sleep, I am thoroughly convinced I may have sleep apnea. Sleep apnea refers to a potentially serious sleep disorder in which breathing repeatedly stops and starts. Some of the symptoms associated with sleep apnea include loud snoring, gasping for air during sleep, awakening with a dry mouth, morning headache, insomnia, excessive daytime sleepiness and irritability. Lucky for me, I experience almost all of these symptoms on a day-to-day basis. The cause? Working a fulltime night shift for almost two years. Personally, I didn’t think there would be a link between night shifts and sleep apnea, however, a study published by the Journal of Physiology and Pharmacology demonstrated that night work significantly increased several breathing variables. With this in mind, my body is still dealing with the aftermath of being on an inverted schedule for so long.
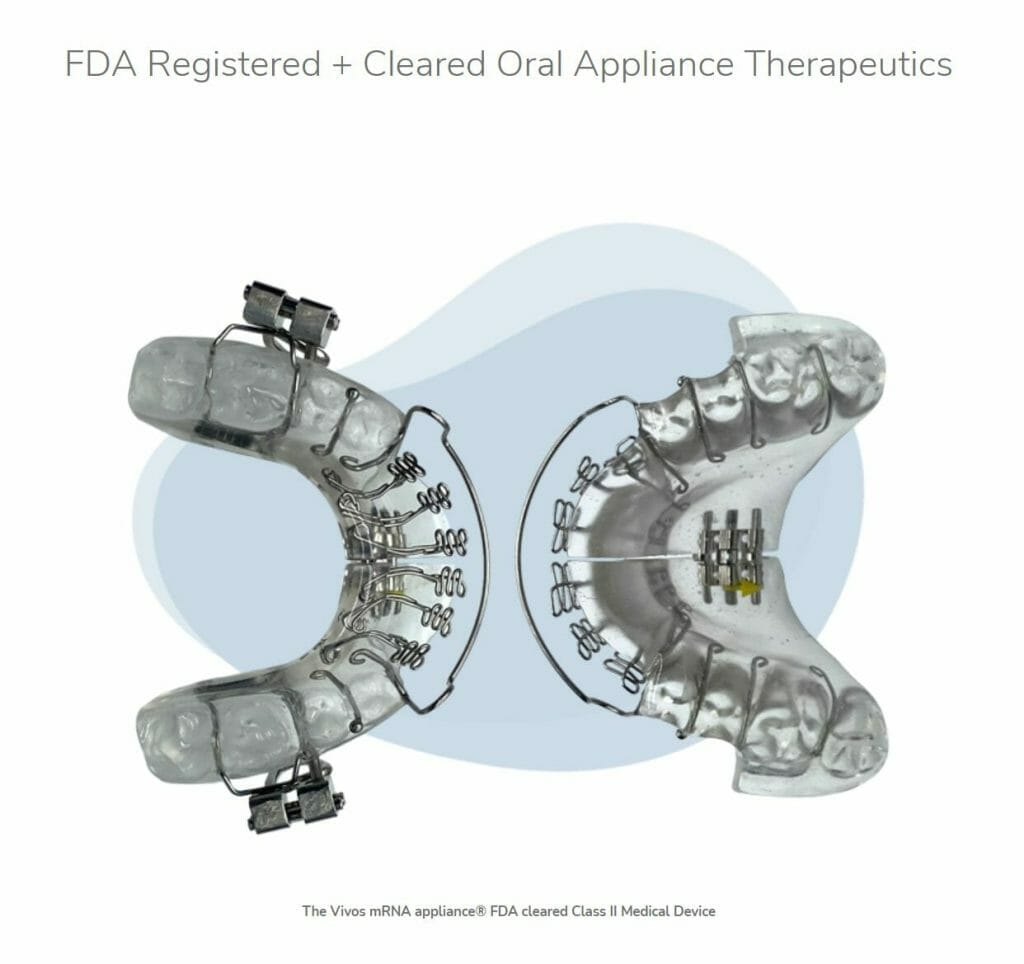
Why haven’t I been tested? In order to test for sleep apnea, an evaluation often involves overnight monitoring at a sleep center. Keep in mind, I have never been good at falling asleep near or around people who can see me. Home testing is an option, however, you’d still need to have someone watch you sleep, like a significant other. As for me, I am as single as they come so that option doesn’t work for me. Even without getting tested, the likelihood of having sleep apnea is high. After all, more than 1 billion people globally and 54 million Americans suffer from sleep apnea, 80% of whom remain undiagnosed. With this in mind, Vivos’ oral mmRNA appliances address the dental tissue anomalies and malformations associated with OSA. Unlike current standard-of-care interventions, patients treated with the Vivos System typically complete their therapy in 12 to 24 months and, in most cases, do not require lifetime intervention.
“Next-generation products like the mmRNA are vital for allowing medical doctors and dentists to continue pushing forward in their joint mission to give patients a better alternative for effectively treating their OSA. Further, this FDA clearance for the mmRNA enables us to expand commercial insurance reimbursement, soon to include Medicare, making this a more cost-effective solution for patients suffering from OSA,” continued Kirk Huntsman.
For context, a 510(k) indicates that an agency is in agreement with the manufacturer that a medical device is similar to a previously approved product, thereby demonstrating that said device is safe, effective and substantially equivalent to a legally marketed device. In this case, the FDA has granted Vivos 510(k) clearance which enables the Company to market its mmRNA devices. Long story short, Vivos now has a marketable device in the sleep apnea device market, which is expected to reach USD$9.9 billion by 2026.
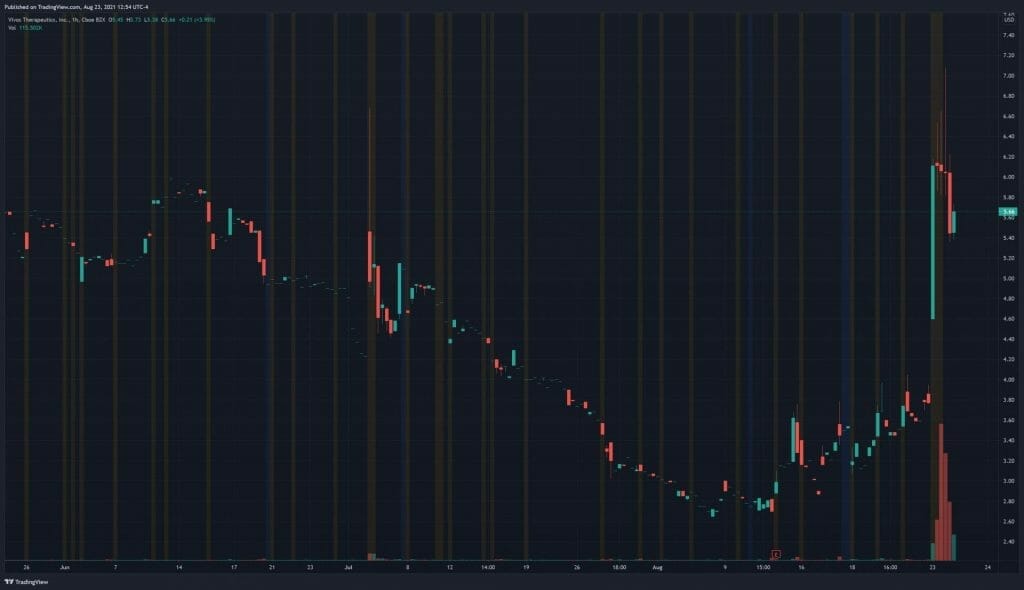
Vivos’ share price opened at $6.52, up from a previous close of $3.84. The Company’s shares are up 47% and are currently trading at $5.69 as of 12:55PM ET.

