PolarityTE (PTE.Q) has provided an update regarding correspondence from the U.S. Food and Drug Administration (FDA) related to the Company’s Investigational New Drug Application (IND) for SkinTE®, which was filed on July 23, 2021.
“In our July 26, 2021, press release regarding the IND submission we described the FDA’s well-established procedures for IND submissions and we believe that the CMC issues FDA has identified are not necessarily unusual for companies working diligently to transition their products from 361 HCT/Ps to 351 HCT/Ps,” commented David Seaburg, Chief Executive Officer.
There are some juicy details to cover here so I am going to cut to the chase. PolarityTE is a biotechnology company focused on discovering, designing, and developing a range of regenerative tissue products and biomaterials for the fields of medicine, biomedical engineering and material sciences. With this in mind, PolarityTE’s SkinTE® is a novel human cellular and tissue-based product derived from a patient’s own skin. The Company filed an IND for SkinTE® last month with a proposed indication for chronic cutaneous ulcers. As defined by the FDA, a chronic cutaneous ulcer refers to a wound that has failed to proceed through an orderly and timely series of events to produce a durable, structural, functional, and cosmetic closure.
According to feedback provided by the FDA, certain Chemistry, Manufacturing, and Control (CMC) items need to be addressed before proceeding with a pivotal study. As a result, the study proposed in PolarityTE’s IND for SkinTE® has been placed on clinical hold. For context, a clinical hold is an order issued by the FDA to the sponsor, in this case PolarityTE, of an IND application to delay a proposed clinical investigation or to suspend an ongoing investigation. The FDA had advised that it will issue a clinical hold letter providing details on the basis for the hold to the Company by September 21, 2021. Normally, I’d chalk this up to just another bad day for a biotech Company, however, PolarityTE’s latest news has investors sweating. Here’s why.
We are actively working to address the issues identified by the FDA and our team plans to respond to the Agency’s feedback as expeditiously as possible, and we are hopeful that we will be able to resolve any open items…we are grateful for FDA’s engagement and eager to advance SkinTE through clinical studies and the regulatory process towards an eventual BLA submission,” continued David Seaburg.
Earlier this month, on August 16, 2021, PolarityTE received a deficiency letter from the Listing Qualifications Department of the Nasdaq Stock Market notifying the Company that, for the preceding 30 consecutive business days, the bid price for the Company’s common stock had closed below the minimum $1.00 per share requirement for continued inclusion on the Nasdaq. In other words, PolarityTE was essentially handed an eviction notice and, unless it is able to regain compliance with the Minimum Bid Price Requirement by February 22, 2022, the Company will be delisted from the Nasdaq. Keep in mind, PolarityTE’s share price dropped 11 cents today following the Company’s latest news. To make matters worse, PolarityTE won’t be able to address the FDA’s clinical hold until it receives a clinical hold letter from the agency on September 21, 2021.
Overall, PolarityTE is in a tight spot, however, the Company has its foot in the right market. In fact, the global chronic wound care market is projected to reach USD$16.36 billion by the end of 2027. In 2019, this market was valued at USD$10.12 billion and was expected to expand at a CAGR of 6.2% between 2020 and 2027. From the start, PolarityTE disclosed that there was the potential for a wide range of outcomes with respect to its SkinTE® IND submission, including but not limited to a clinical hold. With this in mind, the Company is already formulating a plan to address the issues identified by the FDA and resolve CMC issues. If PolarityTE can make SkinTE® fly before the Minimum Bid Price Requirement deadline, the Company could very well make it back to $1.00.
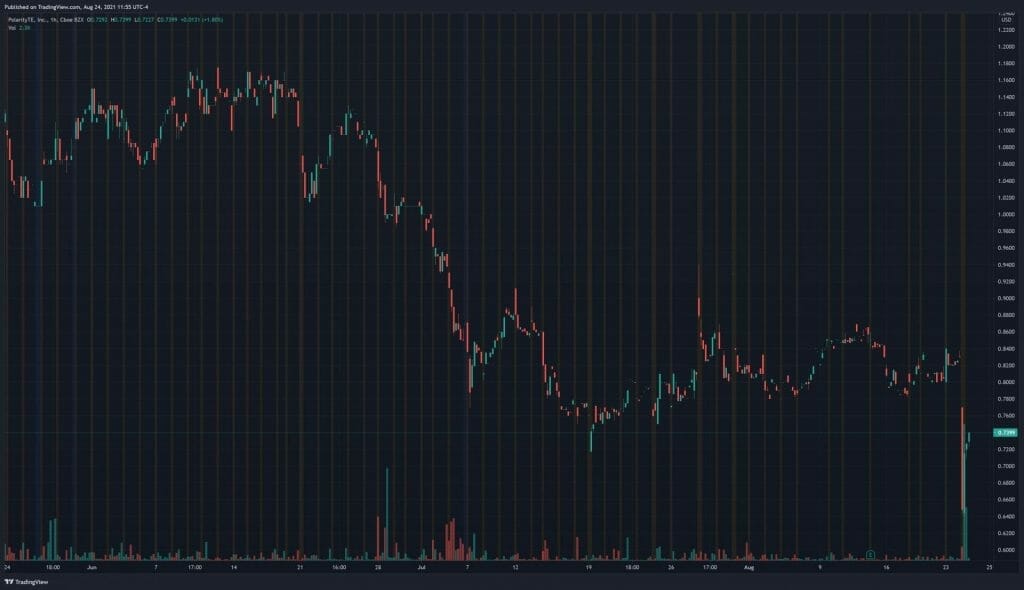
PolarityTE’s share price opened at $0.64, down from a previous close of $0.83. The Company’s shares are down 11% and are currently trading at $0.74 as of 11:55AM ET.

