Xeris Pharmaceuticals (XERS.Q) announces today that the U.S. Food and Drug Administration (FDA) has allowed the Investigational New Drug Application (IND) for the Company’s XeriSol™ levothyroxine (XP-8121) for hypothyroidism to proceed.
“Levothyroxine is one of the most widely prescribed drug products in the United States. However, due to the many challenges associated with oral formulations there remains an area of significant unmet need. We believe that a potentially weekly subcutaneous injection of XeriSol levothyroxine can mitigate many of these challenges and improve compliance, said Kenneth E. Johnson, PharmD, Xeris’ Senior Vice President of Global Development and Medical Affairs.
Xeris Pharmaceuticals is a pharmaceutical company committed to delivering innovative solutions to simplify the experience of https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistering important therapies that people rely on everyday. Utilizing its novel technology platform, the Company is advancing a portfolio of solutions in various therapeutic categories, including its first commercial product, Gvoke® in the U.S. Additionally, Xeris’ proprietary XeriSol™ and XeriJect™ formulation technologies have the potential to offer distinct advantages over conventional product formulations like levothyroxine.
Time for a brief biology lesson. The thyroid gland is responsible for the synthesis, storage, and release of metabolic hormones. These hormones play a pivotal role in regulating critical metabolic processes and are essential for normal growth and development during fetal life, infancy and childhood. With this in mind, levothyroxine is https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered when there is a deficiency of these crucial hormones in the body. Levothyroxine has become the standard for thyroid replacement therapy and is one of the most widely prescribed drug products in the U.S. However, levothyroxine has some shortcomings of its own, namely a narrow therapeutic index. In other words, it would only take a small deviation in dosage to cause a clinically meaningful shift in pharmacological effects when https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered to a patient.
Keep in mind, nearly 40% of patients undergoing treatment with oral levothyroxine are either over-treated or under-treated due to a variety of factors including drug formulation, use of the drug with food, and pre-existing medical conditions, among others. In order to address these issues, Xeris has developed XeriSol™, a formulation technology best suited for peptides and small molecules that encounter formulation challenges, such as levothyroxine. So how does it work?

I could give you the scientific rundown, but an analogy seems more fitting. If you grew up in North America, you have probably heard of Sea-Monkeys before. Sea-Monkeys are basically a “magic” powder that, when added to water, turns into a colony of aquatic shrimp. XeriSol™ essentially follows the same principle by creating a formulation at an optical, specific pH level and processing it into a dried powder. In order to create an injectable solution, this powder is then added to an aprotic, polar liquid, thereby preventing aggregation of the drug substance. However, similar to Sea-Monkeys, when a XeriSol™ formulation is injected into a patient, it is effectively placed back into a water environment where it behaves appropraitely.
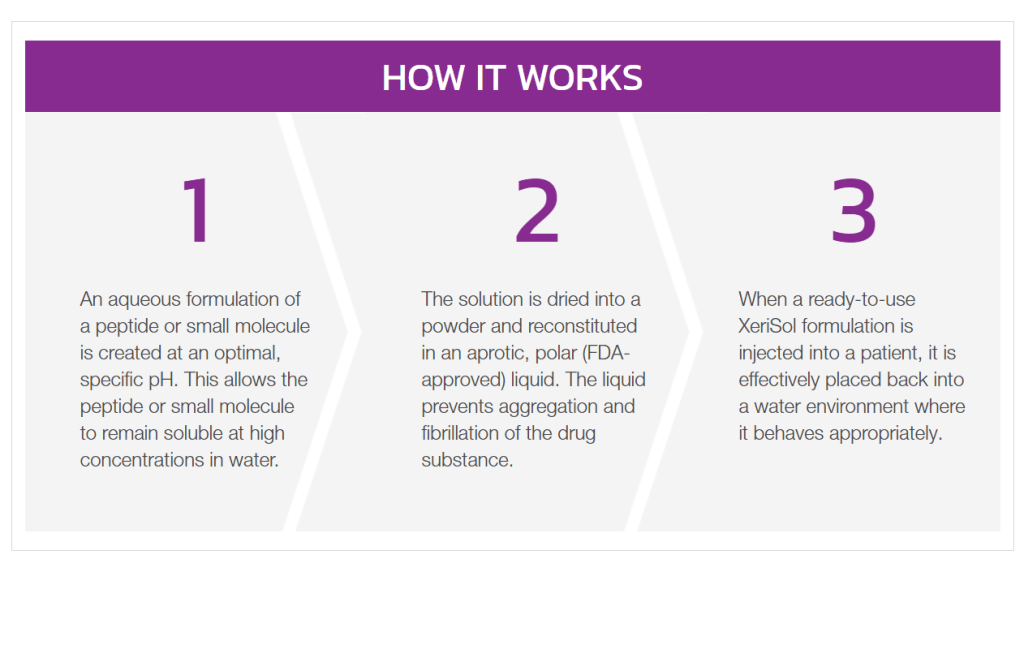
With this in mind, Xeris’ recently approved active IND will enable the Company to initiate a Phase 1 clinical study for XeriSol™ using its novel formulation of levothyroxine for the treatment of hypothyroidism. In addition to Xeris’ latest news, the Company recently announced its Q2 2021 Financial Results. According to these results, Xeris had a cash position of $116 million as of June 30 2021, compared to $133.8 million on December 31, 2021. However, Xeris’ Gvoke® prescriptions topped 21,000 for the first time, growing more than 32% from the previous quarter and 272% year-over-year. Although Xeris’ share price took a bit of a dive following the release of its Q2 2021 results, XeriSol™ appears to be on track to becoming an invaluable asset for the Company, similar to the Gvoke®.
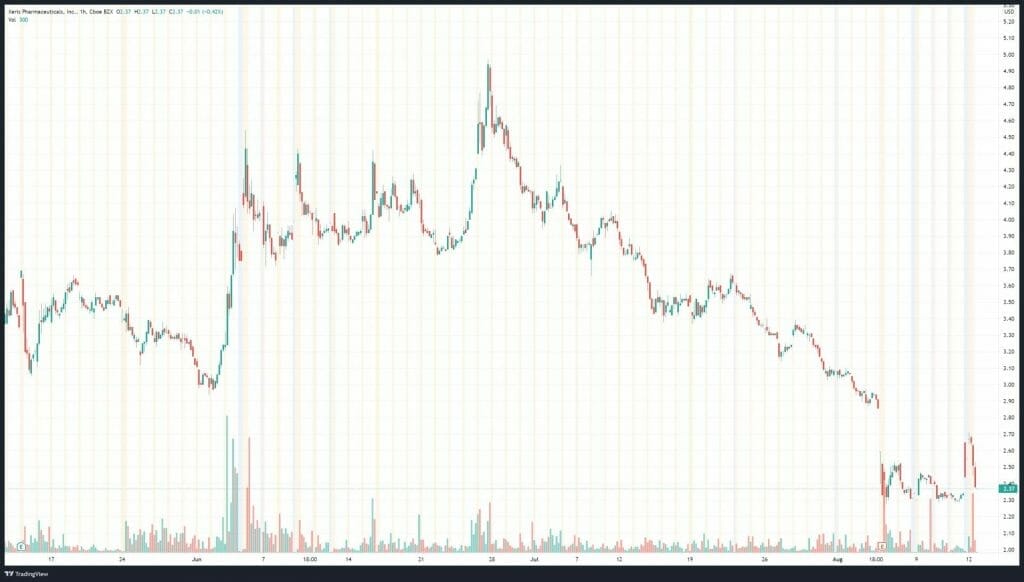
Xeris’ share price opened at $2.60, up from a previous close of $2.35. The Company’s shares are up 1.34% and are currently trading at $2.38 as of 11:09AM ET.

