ERYTECH Pharma (ERYP.Q) announced today that the U.S. Food and Drug Administration (FDA) has granted eryaspase Fast Track designation for the treatment of acute lymphocytic leukemia (ALL) patients who have developed hypersensitivity reactions to E. coli-derived pegylated asparaginase (PEG-ASNase).
“This is yet another significant milestone and meaningful inflection point in advancing our lead product candidate eryaspase, further supporting our recently announced intention to submit a BLA for eryaspase in hypersensitive ALL patients…We believe that the FDA’s Fast Track designation for eryaspase underscores its potential to address this high unmet medical need,” said Gil Beyen, CEO of ERYTECH.
ERYTECH is a clinical stage biopharmaceutical company developing innovative therapies for rare forms of cancer and orphan diseases. Leveraging its proprietary ERYCAPS platform, which encapsulates therapeutic drug substances inside red blood cells, the Company has developed a pipeline of product candidates targeting markets with high unmet medical needs, including the ALL market. ALL is a type of cancer that affects the blood and bone marrow. In people with ALL, the bone marrow becomes incapable of producing a sufficient amount of red cells, white cells and platelets. As a result, people with ALL are more susceptible to anemia, recurrent infections, bruising and bleeding.
To make matters worse, although ALL can occur at any age, it is the most common type of cancer in children in the US and Europe. More than 13,000 cases are diagnosed in the US and Europe each year with most patients being diagnosed before the age of 20. With this in mind, ERYTECH is committed to the development of products that target the altered amino acid metabolism of cancer cells, depriving them of nutrients necessary for their survival. This includes the Company’s lead product candidate, eryaspase, which utilizes L-asparaginase encapsulated inside donor-derived red blood cells to target cancer cells.
Don’t be fooled, asparaginase has nothing to do with asparagus, however, it is associated with ALL treatment. Asparaginase is an enzyme used to treat ALL that starves tumor cells of nutrients, thereby slowing tumor cell growth. Although that sounds fine and dandy, asparaginase is also associated with treatment-limiting hypersensitivity in up to 30% of patients. In other words, some patients experienced adverse reactions when receiving asparaginase treatment, highlighting the need for additional asparaginase based treatment options.
In December 2020, positive results from a Phase 2 trial evaluating eryaspase in primarily pediatric ALL patients who developed hypersensitivity reactions to pegylated asparaginase were presented at the 2020 American Society of Hematology annual meeting. Data demonstrated that eryaspase in combination with chemotherapy provided a sustained asparaginase enzyme activity level, and was generally well tolerated with few hypersensitivity reactions. Having now received Fast Track designation, a program intended to facilitate the expedited development and review of a new drug, eryaspase has entered the fast lane towards commercialization.
According to ERYTECH’s FY2020 financial results, the Company’s total assets were reduced to €80,402,000 and its total liabilities increased to €29,347,000 as of December 31, 2020. Furthermore, ERYTECH’s total cash and cash equivalents at the end of this period were down to €44,446,000 from €73,173,000 year-over-year. Most of the Company’s expenses can be attributed to personnel growth as well as research and development expenses. In particular, R&D expenses grew substantially from €33,468,000 on December 31, 2018 to €57,580,000 on December 31, 2020, with clinical studies accounting for a majority of R&D expenses.
In addition to ERYTECH’s latest news, the Company recently confirmed its intention to submit a Biologics License Application (BLA) for eryaspase in Q4 2021. It is also worth noting that in April 2020, eryaspase was granted Fast Track designation for the development of a second-line treatment for patients with metastatic pancreatic cancer. A Phase 3 trial in this indication completed enrollment in January 2021 and final results are expected in Q4 2021. With this in mind, investors have plenty to look forward to and should certainly keep an eye on ERYTECH.
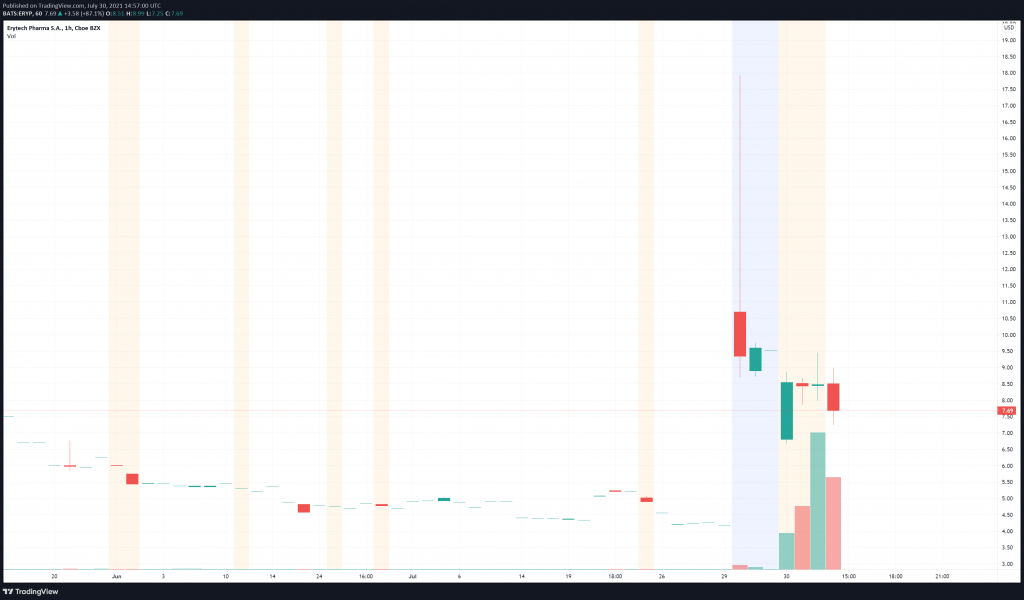
ERYTECH’s share price opened at $4.20, up from a previous close of $4.11. The Company’s shares are up 83% and are currently trading at $7.47 as of 10:58AM ET. This indicates that there has been explosive change following the news.


