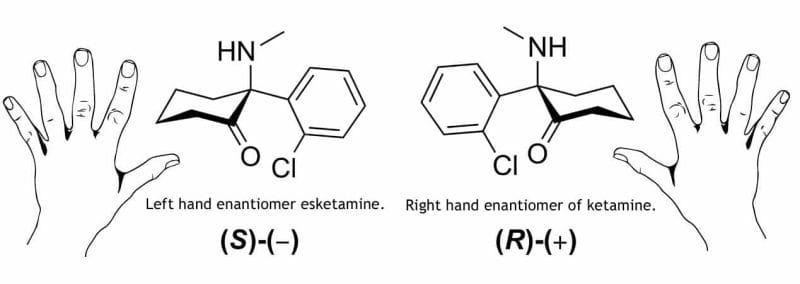
It’s easy to see the word ‘ketamine’ on a press release and assume a company is a full-on psychedelics outfit. But it’s important to understand that even companies who have ketamine in their business plan aren’t all using it the same way. They also aren’t all using the same drug either. This can be somewhat confusing as some companies are using R-ketamine, or generic ketamine, while others are using a ketamine derivative called esketamine.
Esketamine commonly comes in the form of Sparvato and is an FDA-approved ketamine derivative made by big pharma giant Johnson & Jonson (JNJ.Q).
So why is ketamine not approved for depression, but esketamine is?
Patents.
Still underground
Ketamine is generic, meaning that there is no longer patent protection on it and no single pharmaceutical company has exclusive rights to manufacture it. This means there is little incentive for companies to pour money into clinical research into a generic medication.
Ketamine has long been approved in the US as an anesthetic. Meanwhile, outpatient ketamine treatment for depression, along with PTSD, anxiety, OCD, postpartum depression, and bipolar disorder are not approved by the FDA.
Ketamine still has momentum though.
A 2014 study showed that single https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistrations of ketamine were efficacious in the rapid treatment of unipolar and bipolar depression. In these studies, patients with major depressive disorder or bipolar disorder were randomized to received ketamine vs a control. Ultimately ketamine was associated with higher rates of remission relative to saline or midazolam.
Another 2014 study showed among the examined mediators of ketamine’s antidepressant response, only dissociative side effects predicted a more robust and sustained antidepressant. Data from 108 treatment-resistant inpatients meeting criteria for major depressive disorder and bipolar disorder who received a single subanesthetic ketamine infusion were analyzed
A 2017 study showed rats treated with ketamine exhibited a significant reduction in immobility time in comparison with the FSL‐vehicle group. The volumes of the hippocampal CA1.SR and GCL regions were significantly increased 1 day after ketamine treatment in the FSL rats.
Another 2017 study showed people that for people with major depression and clinically significant suicidal ideation, a single subanesthetic ketamine infusion, adjunctive to ongoing pharmacotherapy, was associated with a greater reduction in suicidal thoughts compared with the midazolam control infusion.
Seelos Therapeutics (SEEL.Q) is currently undertaking Phase 2 trials for one of its lead drug candidates SLS-002 for the treatment of acute suicidal ideation and behavior (ASIB) in patients with major depressive disorder (MDD). The trial is looking at the efficacy of a proprietary ketamine intranasal device in the treatment of acute suicidal ideation and behavior (ASIB) in major depressive disorder.
Seelos wants to get ketamine into hospitals for emergency room doctors to use for patients who are suicidal. People will often use the emergency room as a last-ditch place to go when they have nowhere else. There is no approved medication on hand for them to use to calm someone down when they are in a suicidal state. They are kept for surveillance and likely admitted to a psych ward. Ketamine’s proven ability to quickly reduce depressive symptoms seems like a good fit, and Seelos are the only ones going down this road.
While ongoing treatment for suicidal behavior is important, short-term relief is arguably equally as important as suicide is often impulsive and not well thought out at the moment. If a doctor was to https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngister something like ketamine in an emergency room and then the patient went and committed suicide the doctor would be legally liable. There is a also lack of education around ketamine therapy in the medical community, even though studies show its efficacy in treating depression. Usually in these scenarios where old laws hold back scientific research is where real opportunities are found.
Big pharma giant Johnson & Johnson jumped at the opportunity. And because ketamine’s patent ran out, they went for the next best thing. Esketamine.
Johnson & Johnson
Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, filed a patent for esketamine in 2013, and then funded research on the drug. In both sets of trials, Janssen found that depression symptoms reduced just 24 hours after receiving the first dose, and more than 40% of patients went into remission over the course of four weeks. The FDA approved esketamine in 2019.
“Spravato is the first approved antidepressant medication that’s been able to demonstrate a reduction in symptoms of major depressive disorder within 24 hours after the first dose,” says Dr. Michelle Kramer, a psychiatrist and vice president of U.S. neuroscience, medical affairs at Janssen Pharmaceuticals, which makes the drug. Janssen is part of Johnson & Johnson.
There were still questions after the trials though, like why was 34% of the placebo group also in remission, and, why are there no studies involving esketamine vs. ketamine to treat depression. Patients in the studies were also hospitalized for at least several days, received other antidepressants, and attended twice-weekly therapy sessions for four weeks.
Although the drug comes in the form of a nasal spray, it is meant to be given in a hospital or clinic setting, not at home. Esketamine, like ketamine, can cause changes in blood pressure and heart rate, as well as out-of-body experiences for an hour or so after it is https://e4njohordzs.exactdn.com/wp-content/uploads/2021/10/tnw8sVO3j-2.pngistered.
Medical ketamine clinics have been treating patients in the United States since 2014, and there are now dozens of such clinics across the country with more and more popping up as they are insanely profitable as I have written about previously.
There is some red tape.
Patients with MDD who, despite trying at least two antidepressant treatments given at adequate doses for an adequate duration in the current episode, have not responded to treatment are considered to have treatment-resistant depression are then eligible for esketamine. The intention is that esketamine provides rapid relief from depression symptoms until the other medication takes effect.
The current state of this ketamine sector is an improvement from a decade ago, but there is still a long way to go. Esketamine is not nearly as widely available as generic ketamine, and its price has been driven up by Johnson & Johnson’s monopoly on the product. In order to get ketamine more widely available to doctors and end-users more research will be needed. But if Johnson & Johnson was able to move the needle after their product was FDA approved in 2019, maybe another company can do the same for ketamine.

