Tryp Therapeutics (TRYP.C) announced they plan to conduct a Phase 2a clinical trial with the Chronic Pain & Fatigue Research Center at the University of Michigan Medical School.
The study will take place in the Department of Anesthesiology and will focus on evaluating the efficacy of TRYP-8802, Tryp’s oral formulation of synthetic psilocybin, in tandem with psychotherapy for treating fibromyalgia.
Fibromyalgia is a disorder characterized by widespread musculoskeletal pain. Researchers believe that fibromyalgia amplifies painful sensations by affecting the way your brain and spinal cord process painful and nonpainful signals. The disease can cause fatigue, widespread pain, and cognitive difficulties. Fibromyalgia affects 4 million adults in the US.
“Existing treatment options for fibromyalgia are often ineffective and show significant side effects,” said Daniel Clauw, M.D., Director of the Chronic Pain & Fatigue Research Center. “Kevin Boehnke and I are excited to be working with the team at Tryp Therapeutics, who have shown exceptional scientific rigor in their approach to evaluate a new treatment paradigm for the millions of patients suffering from fibromyalgia and other pain-related indications.”
While most psychedelic companies have focused on mental health issues, such as depression, anxiety, PTSD, alcohol abuse disorder, etc., by targeting fibromyalgia, Tryp has decided to focus on the treatment of a bodily issue over a mental one. There are several advantages to doing so, the first and foremost being that you get much better research when you are studying physical ailments. The placebo effect is especially tricky for researchers studying mental health issues, and so targeting an ailment like fibromyalgia will allow Tryp to conduct solid research that governing bodies like the FDA prize.
There are downsides too, however. For instance, fibromyalgia already can already be effectively treated and managed, according to the CDC. In their press release, Tryp says “with available treatment options often proving ineffective, nearly 30% of fibromyalgia patients alarmingly resort to opioid-based medications in an attempt to address symptoms of pain stemming from the disease.” In a sense though, they are giving up the game here, because the reader can infer that ~70% of people with fibromyalgia are receiving effective treatment, and the other 30% are still receiving a treatment, even if people worry about that treatment’s side effects.
The mental health problems other psychedelic companies certainly have treatments that sometimes work, but its undeniable that a huge swath of people with these issues are not being served at all. That creates a huge opening for psychedelic companies to argue that their treatment can help those who otherwise wouldn’t receiving effective treatment.
“We are thrilled to collaborate with such forward-looking clinicians and scientists to develop additional treatment options for fibromyalgia,” commented Jim Gilligan, Ph.D., President and Chief Science Officer at Tryp Therapeutics. “The Chronic Pain & Fatigue Research Center at the University of Michigan brings incomparable experience with evaluating treatments for fibromyalgia and other chronic pain indications, and there is nothing more important to our collective team than creating therapies that will address the daily distress of these patients.”
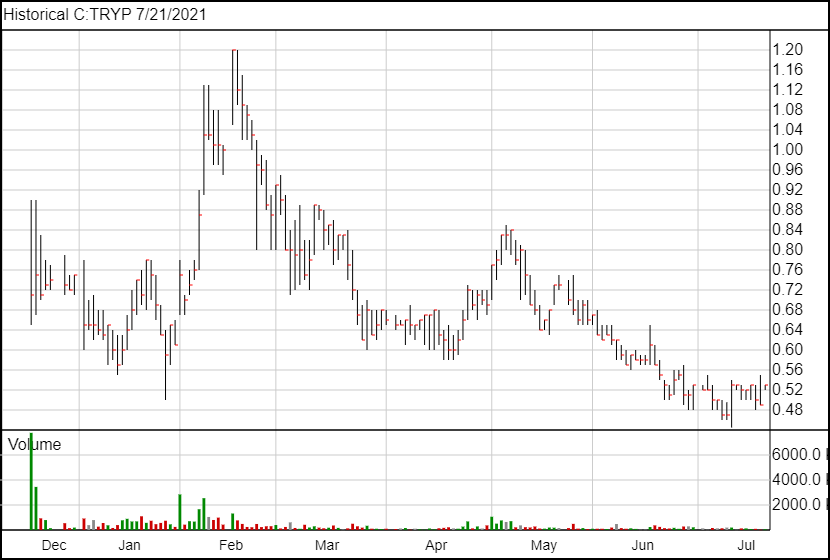
Following the news, TRYP shares are up 4 cents and are currently trading at $0.49.