MediWound (MDWD.Q), a biopharmaceutical company focused on developing solutions for tissue repair and regeneration, today announced positive topline results from its pivotal phase 3 pediatric clinical study (CIDS – Children Innovation Debridement Study) of NexoBrid® to treat children with severe thermal burns.
“We are thrilled to see such robust results across all primary endpoints, which corroborate the positive results of our pivotal phase 3 clinical studies in adult patients, and clearly demonstrate the significant beneficial impact NexoBrid has on the lives of pediatric burn patients…It is gratifying to know that NexoBrid, with these highly compelling top-line results, is one step closer to becoming available as a treatment option for pediatric patients with severe burns,” said Sharon Malka, Chief Executive Officer of MediWound.
We don’t often see headlines related to the burn care treatment sector, yet this market was valued at USD$2.1 billion in 2020. Moreover, the global burn care market is expected to grow at a CAGR of 6.9% from 2021 to 2028, in relation to a a rising incidence rate of burns and an increasing demand for treatment options. One such treatment option includes MediWound’s NexoBrid®, the Company’s topically-applied product for the removal of eschar.
Eschar & Burn Prevalence
Eschar refers to dead tissue that sheds or falls off from the skin, characterized by a dark and often crusty lesion. Although eschars are commonly associated with pressure ulcer wounds, they may also develop following a serious burn injury. With this in mind, a journal published by MDPI Open Access Journals estimates that approximately one-third of burn injuries in the US occur in the pediatric population. Although the treatment of burn injuries between children and adults remains mostly similar, the dermal layer of skin is generally thinner in neonates, infants and children. As a result, various complications arise when treating burns in the pediatric population.
NexoBrid® Phase 3 Study Results
MediWound’s Phase 3 study enrolled 145 pediatric patients across 36 burn centers worldwide and was intended to evaluate the efficacy and safety of NexoBrid® compared to the current standard-of-care (SOC). Overall, the study met its three primary endpoints with a high degree of statistical significance. In particular, NexoBrid® demonstrated a significant reduction in the time needed to complete eschar removal. Furthermore, NexoBrid® was able to reduce the wound area requiring surgical excision while demonstrating non-inferiority to SOC in quality of scars. MediWound’s Phase 3 study also achieved multiple secondary endpoints including reduced blood loss during the eschar removal process.
“This study is one of the most comprehensive randomized controlled studies ever conducted in burn care generally and within the pediatric population specifically…The current mode of pediatric burn management requires intensive medical therapy, which poses challenges due to the surgical complexities in treating young patients with severe burns. Having NexoBrid as a non-surgical option provides a minimally invasive alternative to the current surgical standard of care for treating severe burns in pediatric patients,” commented Dr. Lior Rosenberg, MediWound’s Chief Medical Technology Officer.
Financially, MediWound looks pretty good. According to MediWound’s Q1 2021 results, the Company was able to increase its revenues by 32% from $4.4 million in Q4 2020 to $5.8 million in Q1 2021. Impressively, revenue generated from MediWound’s products and licenses grew 300%, primarily driven by the procurement of NexoBrid® and sales increases outside of the US. On the other hand, the Company cash and short-term investments were reduced to $17.9 million compared to $21.6 million as of December 31, 2020, however, this is most likely the result of increased research and development expenses. With this in mind, MediWound may be worth keeping an eye on as the Company continues to push NexoBrid® as a non-surgical option for the treatment of children with thermal burns.
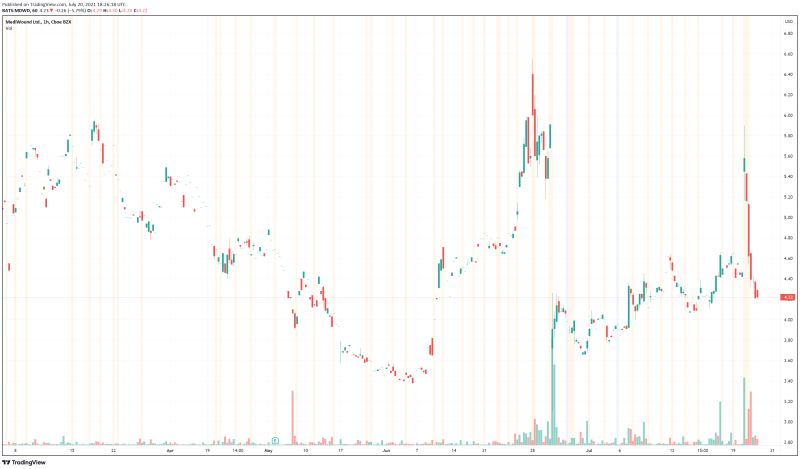
MediWound’s share price opened at $4.94, up from a previous close of $4.90. The Company’s shares are down -5.902% and are currently trading at $4.22 as of 2:27PM ET.

