Revive Therapeutics (RVV.C) has provided an update on the Company’s US Food and Drug Administration (FDA) Phase 3 clinical trial to evaluate the safety and efficacy of bucillamine in patients with mild to moderate COVID-19.
“We are pleased with the status of our phase 3 study in COVID-19 with the aim to seek EUA approval from the FDA for bucillamine in the treatment of mild to moderate COVID-19 patients. We have made tremendous progress over the last few months by engaging over 40 clinical sites and completing patient enrolment to meet its completed and future DSMB interim analysis time points, which will allow for the study to continue and to have potential to seek EUA approval from the FDA…,” said Michael Frank, chief executive officer of the Company.
Bucillamine
Aside from sounding like a pasta, bucillamine is an antirheumatic agent with with a well-known safety profile, commonly prescribed in the treatment of rheumatoid arthritis. However, various studies led by Revive have explored additional applications of bucillamine, including the treatment of gout, a complex form of arthritis. Through a Phase 2a study, conducted in 2015, bucillamine demonstrated safety and efficacy in treating gout in patients with no serious or adverse events.
Additionally, on July 31, 2020, Revive received approval from the FDA to begin its Phase 3 clinical trial to evaluate the safety and efficacy of its Bucillamine in the treatment of patients with mild to moderate COVID-19. With this in mind, Revive’s Phase 3 clinical trial is currently underway, however, the Company announced yesterday that it plans to meet with the Data and Safety Monitoring Board (DSMB) at 600 completed patients, which is expected to occur in Q3 2021.
For context, the DSMB is an independent group of experts that provides its expertise and recommendations for clinical trials such as Revive’s Phase 3 study. As recommended by the DSMB, Revive intends to continue its Phase 3 clinical trial with the suggested 600mg high dose. Moreover, the Company is preparing for the potential filing of an Emergency Use Authorization (EUA) with the FDA in the event that the DSMB recommends Bucillamine for the emergency use treatment of mild to moderate COVID-19.
To date, Revive has partnered with 40 clinical sites in fourteen different states in the US and the Company plans to expand this number to 50. Furthermore, Revive is in discussion with reputable international pharmaceutical companies looking to obtain commercial rights to Revive’s Bucillamine treatment for COVID-19 in Europe, India and Asia. With this in mind, Revive is pursuing a commercialization plan that would leverage the results of its current Phase 3 study.
“…We are continuing to add to our clinical site roster in the U.S. and patient enrollment that would expedite the completion of the Phase 3 study. Also, we are in discussions with international pharmaceutical companies seeking to obtain commercialization rights in various countries around the world,” continued Michael Frank.
If you ask me, Revive is well positioned to capitalize on any positive results generated from its Phase 3 clinical trial. According to Revive’s interim financial results, the Company’s total assets and liabilities were sitting at USD$32,351,131 and $1,011,083, respectively, as of March 31, with cash and cash equivalents of USD$19,023,747. Considering Revive’s Phase 3 clinical trial is continuing without a hitch, the Company’s Bucillamine treatment could find itself on the fast track towards an EUA.
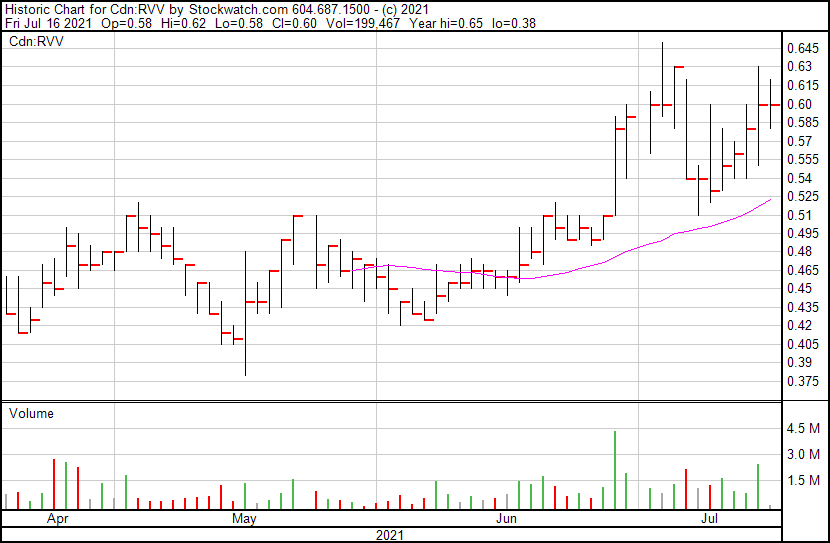
Revive’s share price opened at $0.58, down from a previous close of $0.59. The Company’s shares have returned to and are currently trading at $0.59 as of 11:18AM ET.
Full Disclosure: Revive is a marketing client of Equity Guru.