NLS Pharmaceutics (NLSP.Q) announced today that the U.S. Food and Drug Administration (FDA) has accepted its Investigational New Drug (IND) application for Quilience® extended release, the Company’s lead drug candidate for the treatment of narcolepsy.
“We are pleased to now have an open IND so that we can initiate our clinical program with Quilience®, our novel formulation of mazindol ER, for the treatment of narcolepsy…We remain on track to commence our prospective Phase 2a clinical trial for Quilience® next month as we focus on bringing this treatment option to patients suffering from narcolepsy as soon as possible,” said Alex Zwyer, Chief Executive Officer of NLS.
Pen and Quilience®
Quilience® is NLS’ patented and proprietary formulation of the active compound mazindol. In addition to having a well-established safety record, mazindol has demonstrated efficacy in treating patients suffering from symptoms of narcolepsy. Narcolepsy refers to a chronic neurodegenerative disease caused by a deficiency of orexin-producing neurons in the lateral hypothalamus, the part of the brain that is responsible for an array of cognitive and physical processes. Narcolepsy occurs when there is a profound loss of these orexin producing neurons. This troublesome neurodegenerative disease is characterized by excessive daytime sleepiness (EDS) and an array of symptoms including cataplexy, sleep paralysis and hallucinations.
With this in mind, NLS’ Quilience formulation is intended to treat narcolepsy through the use of partial orexin 2 receptor agonists. According to the Company, a partial orexin 2 receptor agonist may help to replace missing endogenous orexin peptide, addressing the underlying cause of the disease. If approved by the FDA, Quilence would be the only partial orexin 2 receptor agonist as well as the only triple monoamine reuptake inhibitor (TRI) for the treatment of narcolepsy. For context, a TRI is a type of drug that acts as a combined reuptake inhibitor of the monoamine neurotransmitters serotonin, norepinephrine, and dopamine. As a TRI, Quilience is also intended to reduce narcolepsy specific symptoms including EDS and cataplexy attacks.
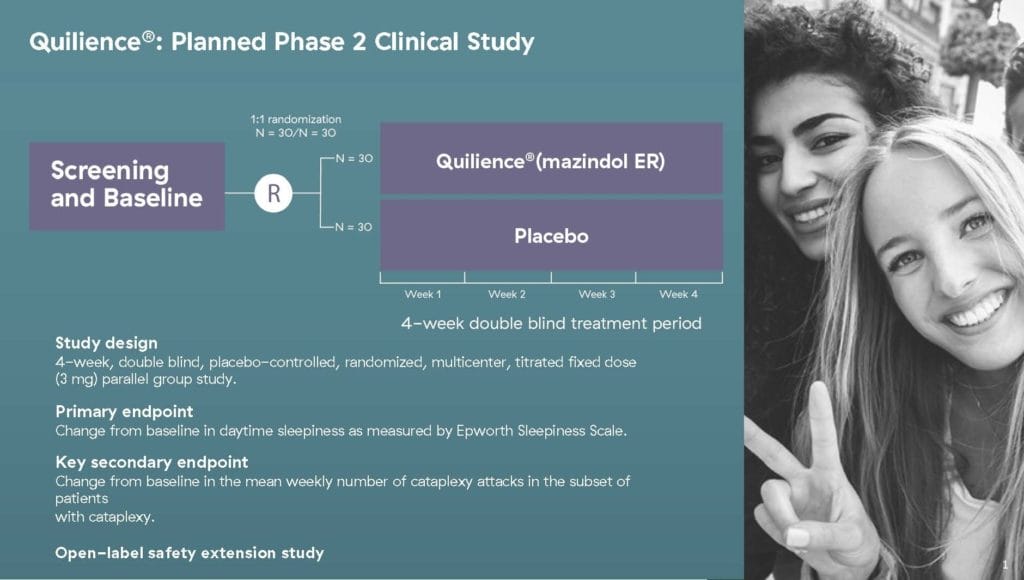
I could drone on forever about Quilience, but I wouldn’t want to risk putting you into a narcoleptic state. Referring back to NLS’ latest news, the accepted open IND will enable the Company to initiate its Phase 2a clinical trial to assess the safety and efficacy of Quilience in patients diagnosed with narcolepsy. The proposed multi-center study will be conducted in both the U.S. and Europe and is expected to enroll 60 patients, commencing in August 2021.
Although NLS’ Quilience looks promising, the Company’s financials aren’t looking as reliable. According to NLS’ 2020 financial results, the Company’s assets were reduced to CHF$233,937 from CHF$362,437 year-over-year. Furthermore, NHS’ liabilities increase to CHF$6,480,558 from $3,912,331 in the same period. From its assets, the Company’s cash and cash equivalents fells to CHF$82,831, down from CHF$213,307. However, the narcolepsy drugs market was estimated at around $2,429 million in 2018, and is expected to reach $5,360 million in 2026, growing at a CAGR of 10.3%. With this in mind, Quilience is a promising new drug candidate that is most certainly worth keeping an eye on. If approved, Quilience could generate some worthwhile returns for NLS and investors alike.
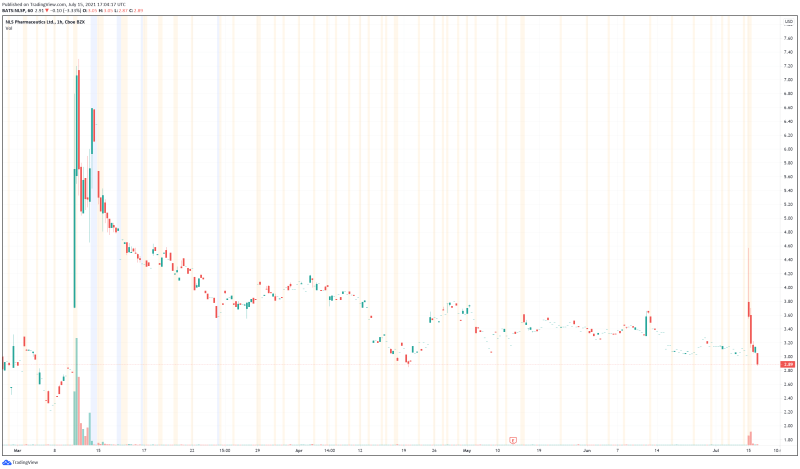
NLS’ share price opened at $3.09, immediately spiking to a high of $3.72. The Company’s shares are down -3.65% and are currently trading at $2.90 as of 1:08PM ET.