Hepion Pharmaceuticals (HEPA.Q) today announced positive topline results from its Phase 2a ‘AMBITION’ NASH clinical trial. Additionally, all primary endpoints of the trial were met.
“In order to embark on a phase 2b program in NASH, these results were critical to inform us on many key parameters for use of CRV431 in this patient population…Despite the challenges of starting clinical research during the COVID pandemic, the team was able to successfully complete this important trial. We look forward to the start of the large Phase 2b ‘ASCEND-NASH’ trial later this year, which will evaluate CRV431 in biopsy-proven NASH subjects with advanced fibrosis,” said Todd Hobbs, MD, Hepion’s Chief Medical Officer.
Who is Hepion Pharmaceuticals?
Hepion Pharmaceuticals (“Hepion”) is a clinical stage biopharmaceutical company focused on the development of targeted therapies for liver disease arising from non-alcoholic steatohepatitis (NASH) and chronic hepatitis virus infection (HBV, HCV, HDV). NASH refers to liver inflammation and damage caused by a buildup of fat in the liver. Typically, people who have a buildup of fat in their liver experience no symptoms or problems. However, in some people this excess of fat can cause inflammation, damage cells in the liver, and impair function.
NASH becomes a serious concern when the liver becomes fibrotic, putting the individual at risk of developing cirrhosis and other complications. Moreover, individuals with advanced liver fibrosis have a significantly higher risk of developing liver cancer. With this in mind, NASH rates are increasing worldwide at an alarming rate due to the spread of the Western diet, obesity and other related conditions. Currently, it is estimated that 4-5% of the global population have NASH, with a higher proportion located in the United States.
CRV431
With this in mind, CRV431 is Hepion’s clinical phase, lead oral drug candidate for the treatment of NASH and viral hepatitis-induced liver disease. CRV431 has demonstrated efficacy in two distinct animal models of NASH and HBV replication. Overall, CRV431 shows promise as an antiviral agent providing protection from cellular stress and death as well as promoting anti-steatotic, anti-inflammatory, anti-fibrotic, and anti-cancer activities.
Referring back to the Company’s AMBITION trial, the primary outcome measure of the trial was the incidence of safety and tolerability events for CRV431 versus placebo. According to results, CRV431 at both study doses (75mg and 225mg soft gel capsules) was well tolerated, and there were no serious adverse events. Moreover, CRV431 blood concentrations after oral dosing were in the anticipate effective range for NASH treatment. This next bit is rather complicated so bear with me.
According to trial results, early indications of efficacy in the form of alanine aminotransferase (ALT) reductions were observed with both CRV431 doses. For context, ALT is an enzyme found mostly in the cells of the liver and kidney. Normally, ALT levels in the blood are low, but when the liver/kidney is damaged, ALT levels increase. Put simply, CRV431 was able to cause a statistically significant reduction in ALT levels, indicating improvement in NASH. With this in mind, CRV431 is poised to become a highly versatile therapeutic drug for the most prominent liver diseases of our time. Currently, no other marketed drugs or NASH drug candidates in development are able to cover all of the same mechanisms as Hepion’s CRV431.
Financials
Looking at Hepion’s Q1 2021 financial results, the Company looks solid. On March 31, 2021, Hepion’s assets were sitting at $123,756,872 million, up from $48,645,253 on December 31, 2020. Furthermore, the Company’s liabilities decreased to $6,182,316 from $8,124,862 in the same period. It is also worth noting that Hepion’s cash by the end of Q1 2021 was a whopping $115,449,085 compared to $16,047,669 year-over-year. With this in mind, Hepion is looking pretty, however, the Company’s share performance is anything but.
As to why Hepion’s stock tanked today, there is no clear answer. As a pharmaceuticals company, Hepion may just be more prone to market volatility than most. With positive trial results and a strong financial position, Hepion’s stock may normalize in the next few days. Regardless, the Hepion’s latest news is good, and the Company undoubtedly shows potential as a cheap biopharmaceuticals company with a pipeline of innovative products. At the very least, Hepion is worth keeping an eye on in the next few days to see if the Company’s stock is able to rebound.
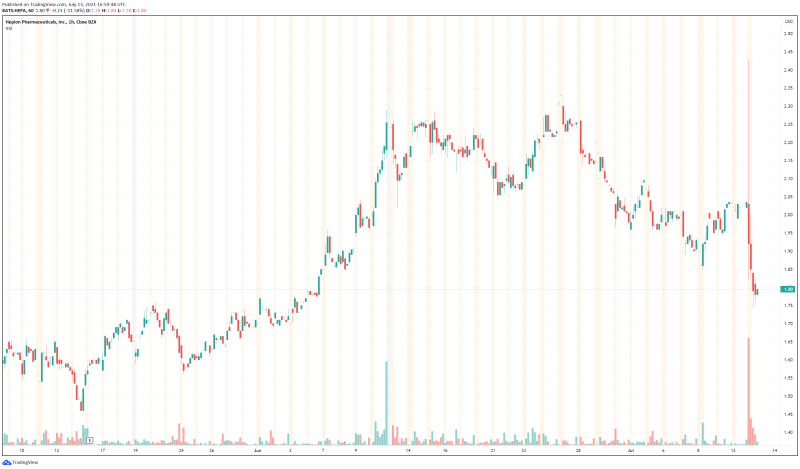
Hepion’s share price opened at $1.95, down from a previous close of $2.03. The Company’s shares are down -11.65% and are currently trading at $1.79 as of 1:01PM ET.


Pingback: What the Hep is Going On with Hepion Pharmaceuticals (HEPA.Q)? – OTC.Watch